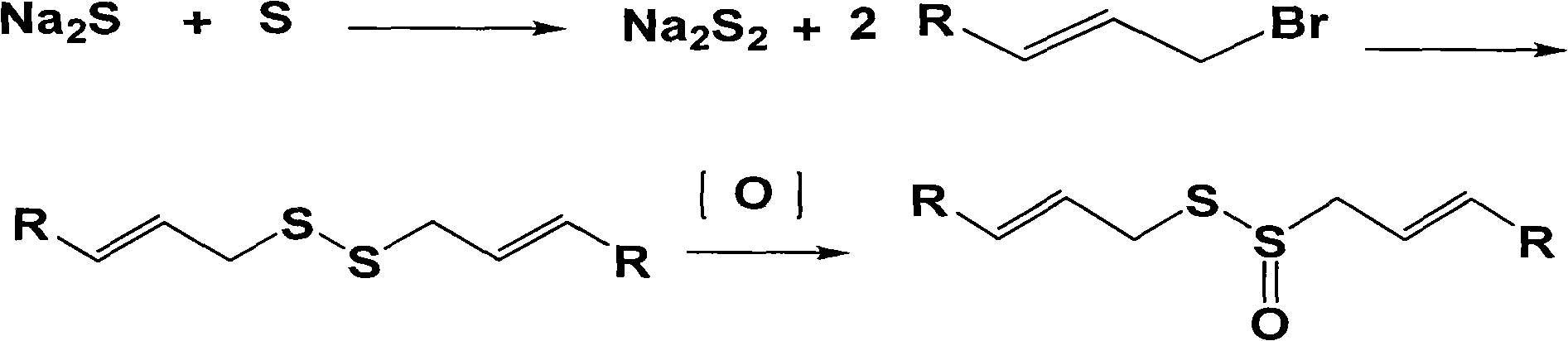Substituted organic sulfur compound and application thereof
A compound and organic sulfur technology, applied in organic chemistry, organic active ingredients, medical preparations containing active ingredients, etc., to achieve high application value, simple and easy production method, and high water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
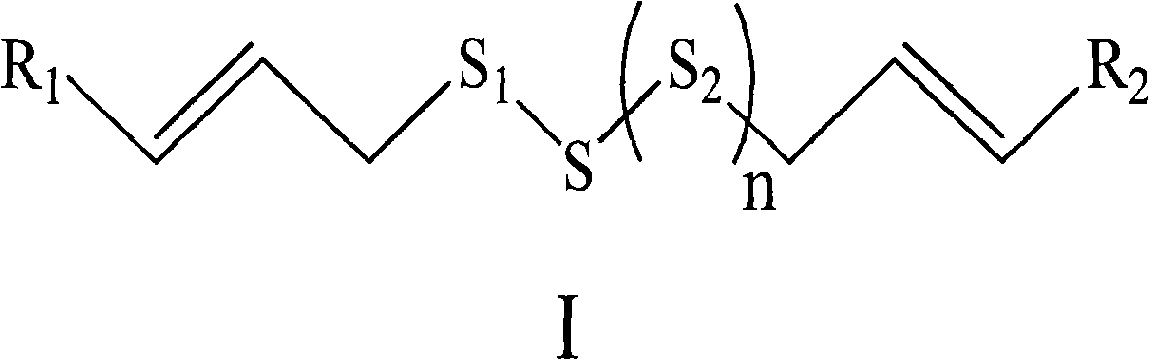
[0043] Example 1: Compound I 1 : Preparation of dicrotonic acid trisulfide
[0044] In a 5000ml four-neck flask, an electric stirrer, a reflux condenser, a thermometer and a constant pressure addition funnel were installed, and 248g (1.0mol) of crystalline sodium thiosulfate pentahydrate and 2000ml of 40% (v:v) ethanol solution were added thereto. , after stirring and dissolving, keep the temperature at 15-25°C, add dropwise 200ml of 40% (v:v) ethanol solution containing 153g (0.9mol) 4-bromocrotonic acid, drop it in about 0.5 hours, and heat up after dropping Stir and react at 40-45°C for 5 hours, after the reaction is complete, cool to room temperature, adjust the pH value to about 7.0-7.5 with 2mol / L ammonia water, add dropwise 250ml of aqueous solution containing 78g (1.0mol) of sodium sulfide, after dropping, The reaction was stirred at room temperature for 0.5 h and left to stand overnight. Use 0.1N HCl to adjust the pH value to about 5.0-5.5, remove ethanol under high...
Embodiment 2
[0060] Embodiment 2: Compound I 2 : Preparation of Dicrotonate Disulfide-Oxygen[S]
[0061] 2.1 Preparation of sodium disulfide solution
[0062] In a 5000ml four-necked flask, an electric stirrer, a reflux condenser, and a thermometer are installed, and 78g (1.0mol) Na 2S 9H 2 O and 2000ml deionized water are added thereto, heated and stirred, and after Na 2S 9H 2 O is completely dissolved, rise to At 80°C, 26g (0.8mol) of elemental sulfur was added, and the reaction was continued for 1 hour at reflux temperature to obtain a reddish-brown sodium disulfide solution.
[0063] 2.2 Preparation of dicrotonate disulfide
[0064] In the sodium disulfide solution prepared above, add 25 g of phase transfer catalyst tetrabutylammonium bromide, stir, get 132 g (0.8 mol) of 4-bromocrotonic acid, be dissolved in the ethanol solution of 180 ml 40% (v: v), Add dropwise to the above-mentioned sodium disulfide solution at a rate of 50ml / h, stir and react at reflux temperature for 6 hours, ...
Embodiment 3
[0077] Embodiment 3: Preparation of dicrotonic acid trisulfide tromethamine salt
[0078] In the three-necked round-bottomed flask, according to the molar ratio, dicrotonic acid trisulfide: tromethamine: methanol = 1: 2: 16 was added in turn, heated and stirred until completely dissolved, cooled in the refrigerator, and the precipitated crystals were sucked and filtered. That is, dicrotonic acid trisulfide tromethamine salt powder crystals were obtained, and determined according to the law.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com