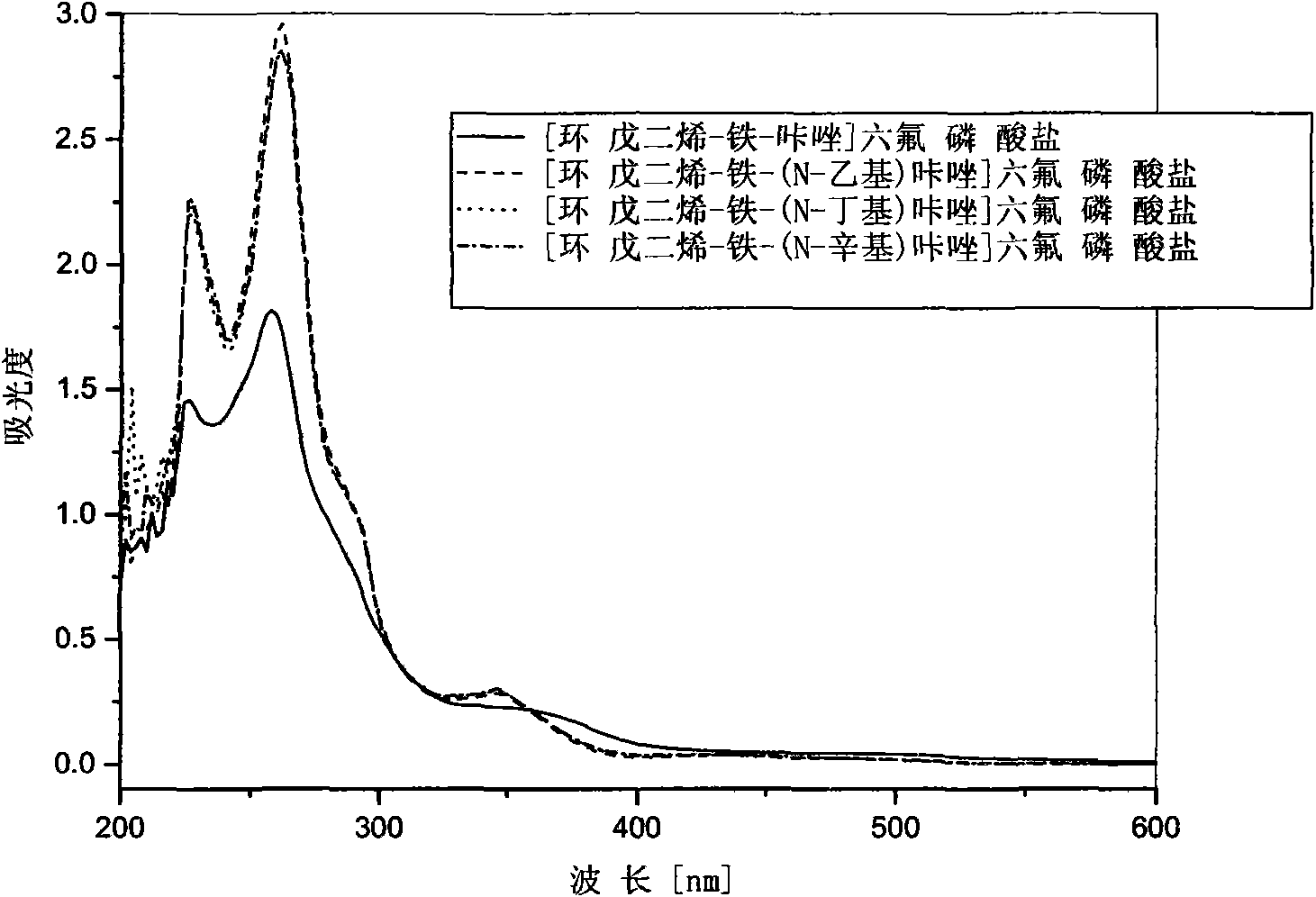
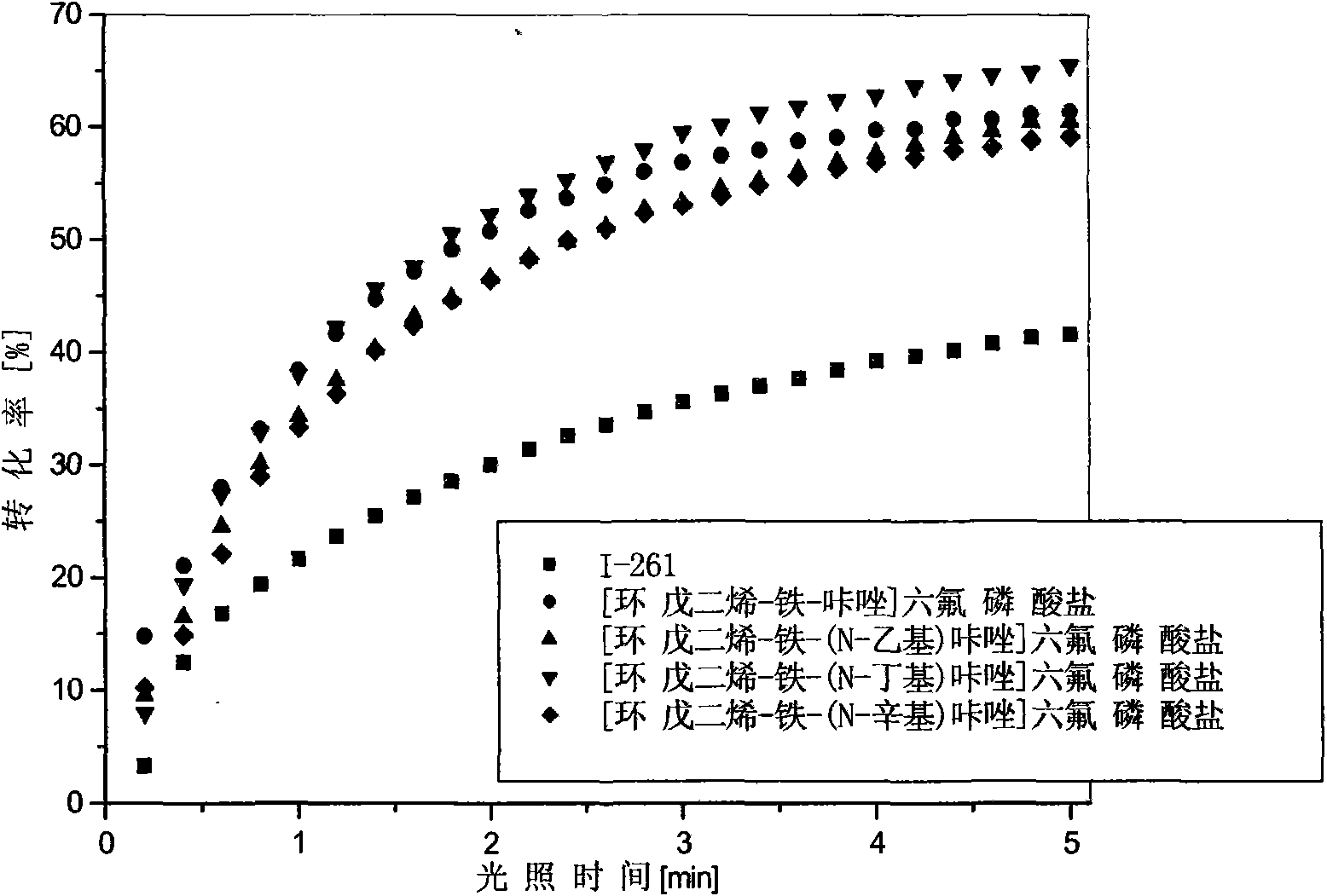
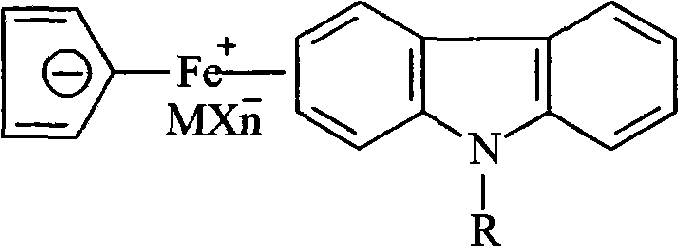Ferrocenium salt photoinitiators containing N-alkyl substituted carbazole ligand and preparation method thereof
A carbazole and alkyl technology, applied in the field of cationic photopolymerization initiators, can solve problems such as inability to match light sources and limit the application of cationic photopolymerization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1 prepares cyclopentadiene-iron-carbazole hexafluorophosphate
[0022] Put 0.025mol ferrocene, 0.0375mol carbazole, 0.075mol anhydrous AlCl into the four-necked flask 3 , 0.025mol Al powder and 50ml cyclohexane to make it stir and dissolve, and pass into N 2 , and then slowly raised the temperature to 84°C and maintained the reflux state for 10h.
[0023] After the reaction liquid is cooled, pour 10% methanol solution into it, stir and hydrolyze, filter, separate liquids, wash the organic phase with water, combine with the water phase, wash repeatedly with cyclohexane, and then add excess KPF 6 The aqueous solution has a yellow precipitate. The solid was dried and purified by column chromatography to obtain the product cyclopentadiene-iron-carbazole hexafluorophosphate.
Embodiment 2
[0024] Embodiment 2 prepares cyclopentadiene-iron-N-ethylcarbazole hexafluorophosphate
[0025] Put 0.025mol ferrocene, 0.0375mol N-ethylcarbazole, 0.075mol anhydrous AlCl into the three-necked flask 3 , 0.025mol Al powder and 50ml cyclohexane to make it dissolve by stirring, pass N 2 Then slowly raise the temperature to 84°C and maintain the reflux state for 10h.
[0026] After the reaction liquid is cooled, pour 10% methanol solution into it, stir and hydrolyze, filter, separate liquids, wash the organic phase with water, combine with the water phase, wash repeatedly with cyclohexane, and then add excess KPF 6 The aqueous solution has a yellow precipitate. The solid was dried and purified by column chromatography to obtain the product cyclopentadiene-iron-N-ethylcarbazole hexafluorophosphate.
Embodiment 3
[0027] Embodiment 3 prepares cyclopentadiene-iron-N-butylcarbazole hexafluorophosphate
[0028] Take 0.0375mol N-butylcarbazole, 0.025mol ferrocene, 0.075mol anhydrous AlCl 3 , 0.025mol Al powder and 50ml cyclohexane were put into a three-necked flask, stirred and dissolved, and passed N 2 Then slowly raise the temperature to 84°C and maintain the reflux state for 10h.
[0029] After the reaction liquid is cooled, pour 10% methanol solution into it, stir and hydrolyze, filter, separate liquids, wash the organic phase with water, combine with the water phase, wash repeatedly with cyclohexane, and then add excess KPF 6 An orange-yellow precipitate appeared in the aqueous solution. The solid was dried and purified by column chromatography to obtain the product cyclopentadiene-iron-N-butylcarbazole hexafluorophosphate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com