Method for in-situ preparation of silver nanoparticles loaded on natural cellulose sheet
A technology of natural cellulose and silver nanoparticles, which is applied in the direction of metal coating, etc., can solve the problems of complex preparation methods, widened particle size distribution, and larger particle size of silver nanoparticles, and achieves a simple preparation process and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The natural cellulose source used is the dust-free wiping paper (Kinwipies) produced by Canada Kimberly-Clark Company. Cutting area is 40×50cm 2 The dust-free wiping paper, dipped in the silver nitrate aqueous solution with a concentration of 100mmol / L, put it still for 1 minute, took it out and immersed it in anhydrous ethanol solution, put it still for 30 seconds, took it out and soaked it in sodium borohydride with a concentration of 800mmol / L Aqueous solution, the reaction is violent, and a large number of bubbles are generated rapidly. The dust-free wipe paper is fixed with a glass rod in the beaker carrying the sodium borohydride reaction solution. It can be observed that the dust-free wipe paper turns from white to yellow, indicating the formation of silver nanoparticles. , put it still for 10 minutes, take it out and soak it in deionized water, take it out after 1 minute, place it in a vacuum dryer, dry it at room temperature for 24 hours, and obtain the silver ...
Embodiment 2
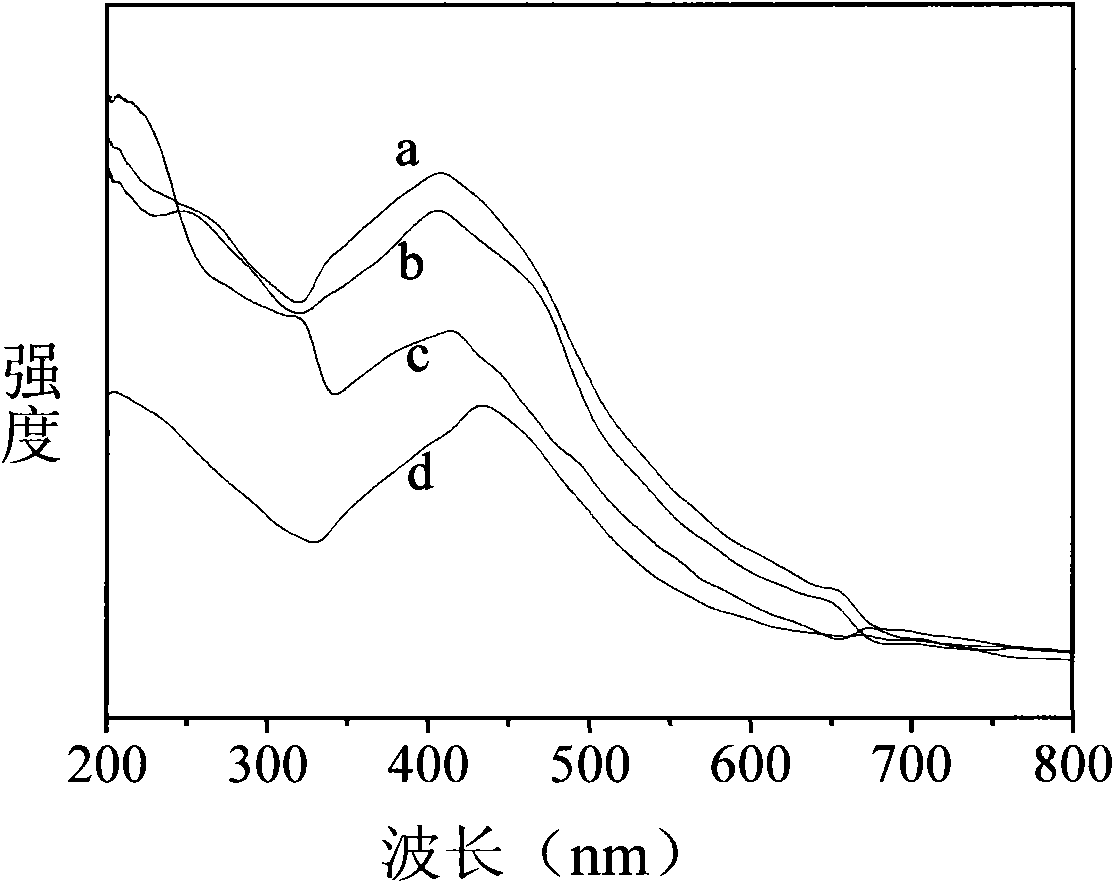
[0034] Implementation step is identical with embodiment 1, but the concentration of silver nitrate aqueous solution becomes 100mmol / L, and the concentration of reducing agent sodium borohydride is successively 200mmol / L, 400mmol / L, 600mmol / L, 800mmol / L, figure 2 The ultraviolet-visible absorption spectrogram of the in-situ load on the dust-free wiping paper produced by Canadian Kimberly-Clark Company produced under the above concentration ratio conditions, wherein curves a, b, c, and d are respectively in The concentration ratio silver nitrate: sodium borohydride is 100: 200mmol / L, 100: 400mmol / L, 100: 600mmol / L, the ultraviolet-visible absorption spectrum of the sample prepared under the condition of 100: 800mmol / L. Depend on figure 2 It can be seen that with the increase of the reducing agent sodium borohydride concentration, the peak position of the curve d, c, b, and a gradually shifts to blue. From the classical theory of the relationship between the peak and the partic...
Embodiment 3
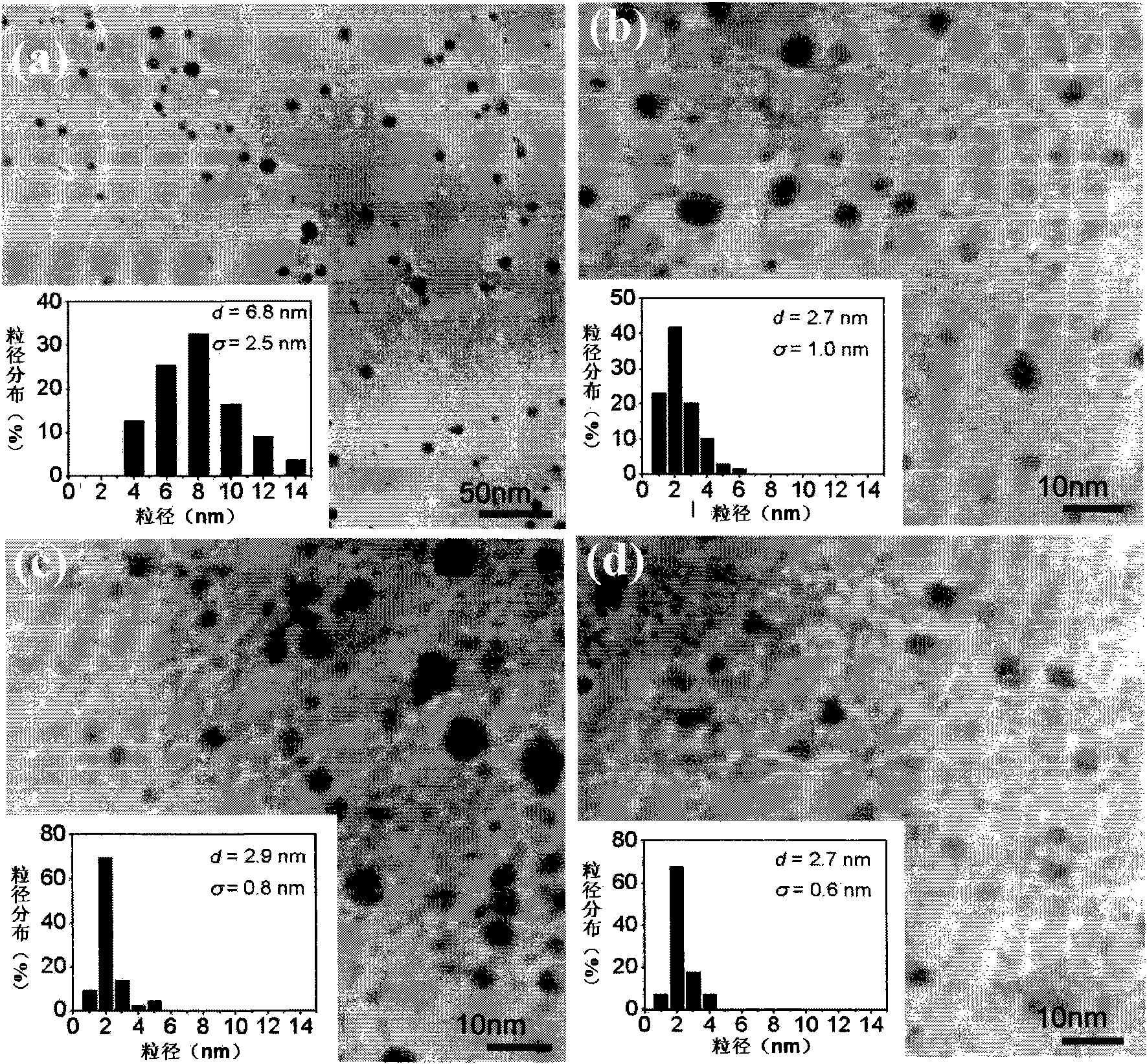
[0037] Implementation steps are the same as in Example 1, but the concentration ratio is changed in a larger range. image 3 (a), 3(b), 3(c), and 3(d) are the in-situ loading of silver nanoparticles on the dust-free wiping paper produced by Canada Kimberly-Clark Company under different concentration ratio conditions. Transmission electron microscope photographs, in which image 3 (a), image 3 (b), image 3 (c), image 3 (d) are transmission electron micrographs of samples prepared under the conditions of molar concentration ratio silver nitrate: sodium borohydride 1:2, 1:8, 1:80, 1:800 respectively. It can be seen from the above figure that when the concentration ratio changes from 1:2 to 1:8, the average particle diameter of the sample obtained changes from 6.8 to 2.7nm, and when the concentration ratio is further increased until 1:800, the average particle diameter of the sample is basically maintained at 2.7nm range, but the particle size distribution of the sample con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| electrical resistivity | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com