Taxol lipid preparation and method for preparing same
A paclitaxel lipid and paclitaxel technology, applied in the field of medicine, can solve the problems of poor stability of liposome targeting system, difficult industrialized production, unsatisfactory targeting distribution, etc., to avoid toxic and side effects, improve safety and compliance, and prepare The effect of simple and easy process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1. Preparation of paclitaxel lipid formulation
[0041] 1. Preparation of paclitaxel lipid preparation
[0042] 1. Weigh 5 g of phospholipid (Lipoid, Germany) and 10 g of propylene glycol, and heat to dissolve in a water bath at 45° C. to obtain solution A.
[0043] 2. Weigh 0.6g paclitaxel, 15g HS15 (German BASF company), 5g glycerin, 0.18g anhydrous citric acid, 65g anhydrous ethanol, and dissolve at room temperature to obtain solution B.
[0044] 3. Mix solution A and solution B, stir at room temperature, mix evenly, and filter to obtain a paclitaxel lipid preparation, which is protected by nitrogen gas and sealed for storage.
[0045] 2. Preparation of paclitaxel nano preparation
[0046] The paclitaxel lipid preparation prepared in step 1 is diluted with 20 times the volume of normal saline to obtain a paclitaxel nano preparation.
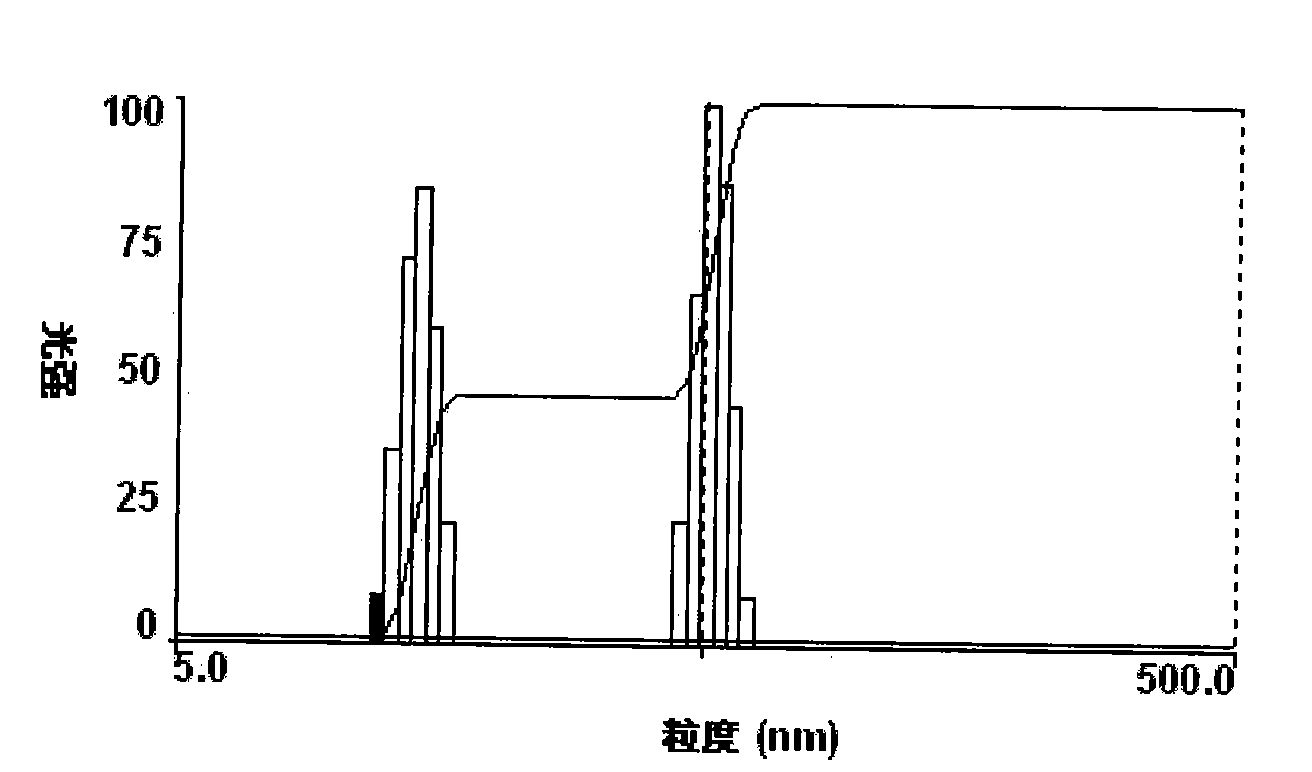
[0047] 3. The particle size determination of paclitaxel nano preparation
[0048] The particle size of the paclitaxel nano-preparation was ...
Embodiment 2
[0049] Example 2. Preparation of paclitaxel lipid formulation
[0050] 1. Preparation of paclitaxel lipid preparation
[0051] 1. Weigh 20 g of phospholipids and 10 g of absolute ethanol, and heat to dissolve in a water bath at 55°C to obtain solution A.
[0052] 2. Weigh 0.6g paclitaxel, 50g HS15, 0.18g anhydrous citric acid, 20g anhydrous ethanol, and dissolve at room temperature to obtain solution B.
[0053] 3. Mix solution A and solution B, stir at room temperature, mix evenly, and filter to obtain a paclitaxel lipid preparation, which is protected by nitrogen gas and sealed for storage.
[0054] 2. Preparation of paclitaxel nano preparation
[0055] The paclitaxel lipid preparation prepared in step 1 is diluted with 20 times the volume of water for injection to obtain paclitaxel nano preparation.
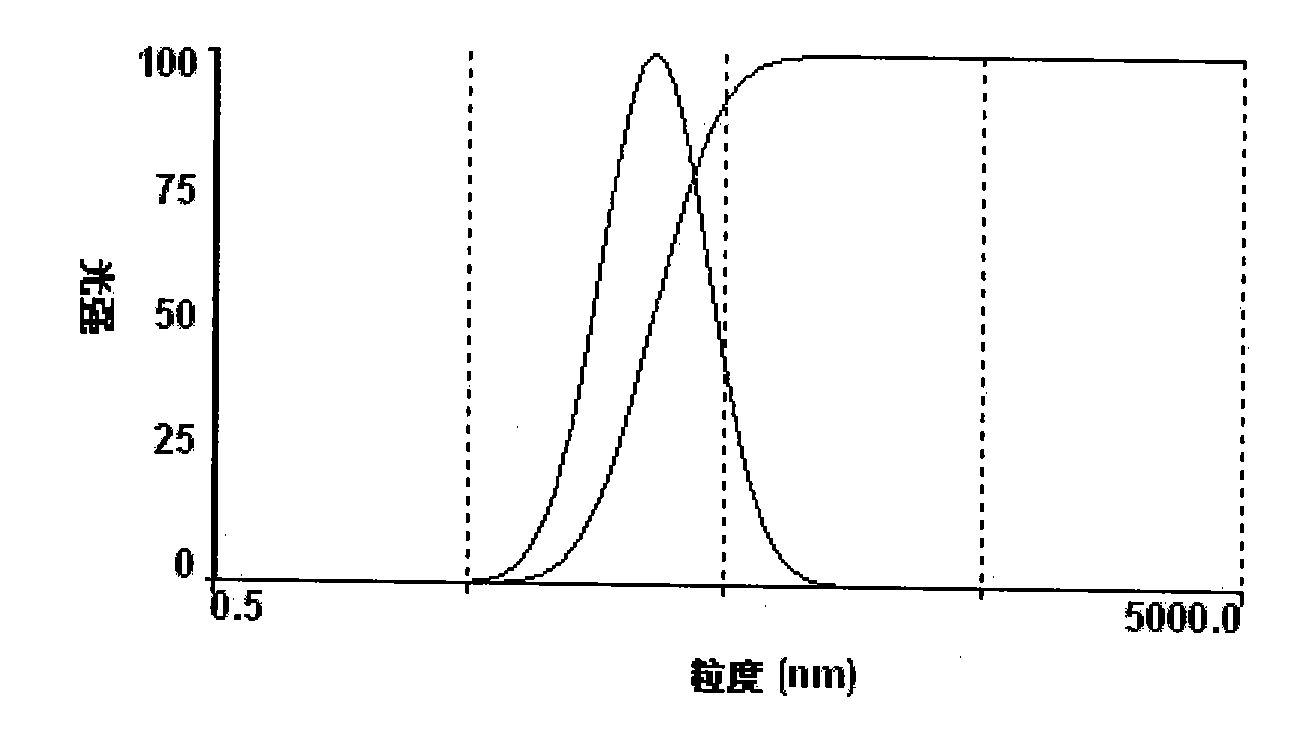
[0056] 3. The particle size determination of paclitaxel nano preparation
[0057] The particle size of the paclitaxel nano-preparation was measured with the ZetaPALS laser dynamic light sc...
Embodiment 3
[0058] Example 3. Preparation of paclitaxel lipid formulation
[0059] 1. Preparation of paclitaxel lipid preparation
[0060] 1. Weigh 10g of phospholipid and 10g of propylene glycol, and heat to dissolve in a water bath at 50°C to obtain solution A.
[0061] 2. Weigh 0.6g paclitaxel, 36g HS15, 15g glycerol, 0.18g anhydrous citric acid, 29g anhydrous ethanol, and dissolve at room temperature to obtain solution B.
[0062] 3. Mix solution A and solution B, stir at room temperature, mix evenly, and filter to obtain a paclitaxel lipid preparation, which is protected by nitrogen gas and sealed for storage.
[0063] 2. Preparation of paclitaxel nano preparation
[0064] The paclitaxel lipid preparation prepared in step 1 is diluted with 20 times the volume of normal saline to obtain a paclitaxel nano preparation.
[0065] 3. The particle size determination of paclitaxel nano preparation
[0066] The particle size of the paclitaxel nano-preparation was measured with the ZetaPALS laser dynamic l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com