A sustained-release preparation for treating Alzheimer's disease and its preparation method
A slow-release preparation and slow-release material technology, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pill delivery, etc. It can solve the problems of low bioavailability, low blood drug concentration, and small AUC, etc. problem, achieve the effect of avoiding burst release effect and high physiological tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
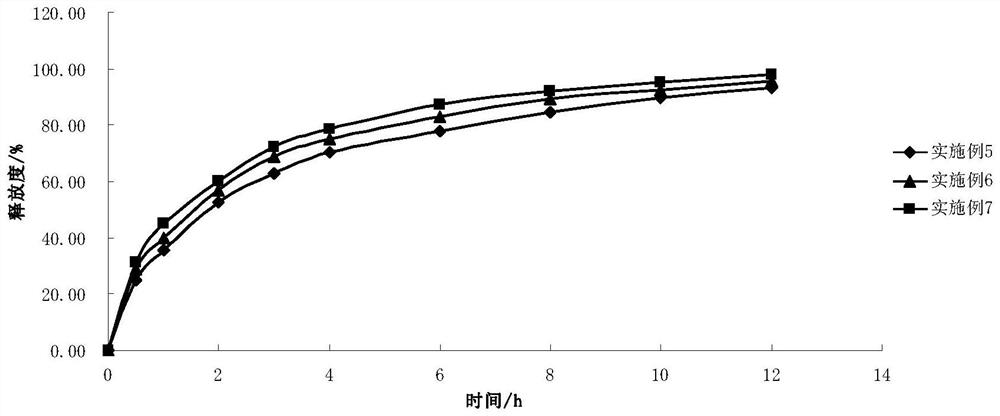
Examples
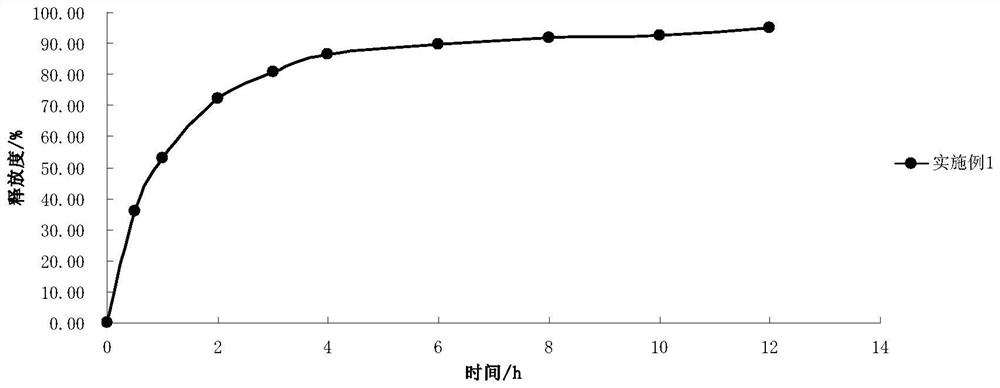
Embodiment 1
[0033] Embodiment 1: the prescription of 2-(4-methylthiazol-5-yl) ethyl nitrate hydrochloride slow-release preparation is as follows:
[0034] Dosage of ingredients:
[0035]
[0036]
[0037] Taking the preparation of 10,000 tablets of 2-(4-methylthiazol-5-yl)ethyl nitrate sustained-release preparation as an example, all excipients were pulverized respectively and passed through a 100-mesh nylon sieve. Take the main ingredient and grind it into powder. Take by weighing 499.5g 2-(4-methylthiazol-5-yl) ethyl nitrate hydrochloride, 750.0g glyceryl behenate, 235.5g microcrystalline cellulose PH101 respectively according to the prescription composition in embodiment 1, mix Evenly, use 4% povidone ethanol solution to make soft material, granulate with 18 mesh sieve, dry at 50°C, granulate with 20 mesh sieve, add 15.0g magnesium stearate, mix evenly, and press with φ7 punch to tablet. The sustained-release preparation of 2-(4-methylthiazol-5-yl)ethyl nitrate hydrochloride wa...
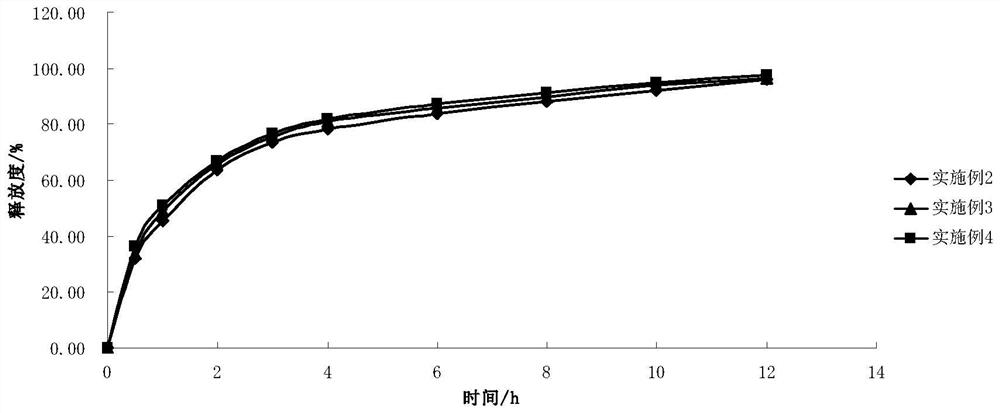
Embodiment 2
[0046] Embodiment 2: the prescription of 2-(4-methylthiazol-5-yl) ethyl nitrate hydrochloride slow-release preparation is as follows:
[0047] Dosage of ingredients:
[0048]
[0049] Taking the preparation of 10,000 tablets of 2-(4-methylthiazol-5-yl)ethyl nitrate sustained-release preparation as an example, all excipients were pulverized respectively and passed through a 100-mesh nylon sieve. Take the main ingredient and grind it into powder. Take by weighing 499.5g 2-(4-methylthiazol-5-yl) ethyl nitrate hydrochloride, 120.0g glyceryl behenate, 180.0g hydroxypropyl methylcellulose respectively according to the prescription composition in Example 2 K100M, 685.5g microcrystalline cellulose PH101, mix well, make soft material with 4% povidone ethanol solution, granulate with 18 mesh sieve, dry at 50°C, granulate with 20 mesh sieve, add 15.0g magnesium stearate , mix well, and press the φ7 punch into tablets. The sustained-release preparation of 2-(4-methylthiazol-5-yl)eth...
Embodiment 3
[0054] Embodiment 3: the prescription of 2-(4-methylthiazol-5-yl) ethyl nitrate hydrochloride slow-release preparation is as follows:
[0055] Dosage of ingredients:
[0056]
[0057] Taking the preparation of 10,000 tablets of 2-(4-methylthiazol-5-yl)ethyl nitrate sustained-release preparation as an example, all excipients were pulverized respectively and passed through a 100-mesh nylon sieve. Take the main ingredient and grind it into powder. Take by weighing 499.5g 2-(4-methylthiazol-5-yl) ethyl nitrate hydrochloride, 120.0g glyceryl behenate, 300.0g hydroxypropyl methylcellulose respectively according to the prescription composition in Example 3 K15M, 685.5g microcrystalline cellulose PH101, mix well, make soft material with 4% povidone ethanol solution, granulate with 18 mesh sieve, dry at 50°C, granulate with 20 mesh sieve, add 15.0g magnesium stearate , mix well, and press the φ7 punch into tablets. The sustained-release preparation of 2-(4-methylthiazol-5-yl)ethy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com