Method for preparing alpha-isophorone
A technology of isophorone and acetone, applied in the field of compound preparation, can solve the problems of easy deactivation of gas-phase catalysts, low reaction efficiency per unit volume reactor, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
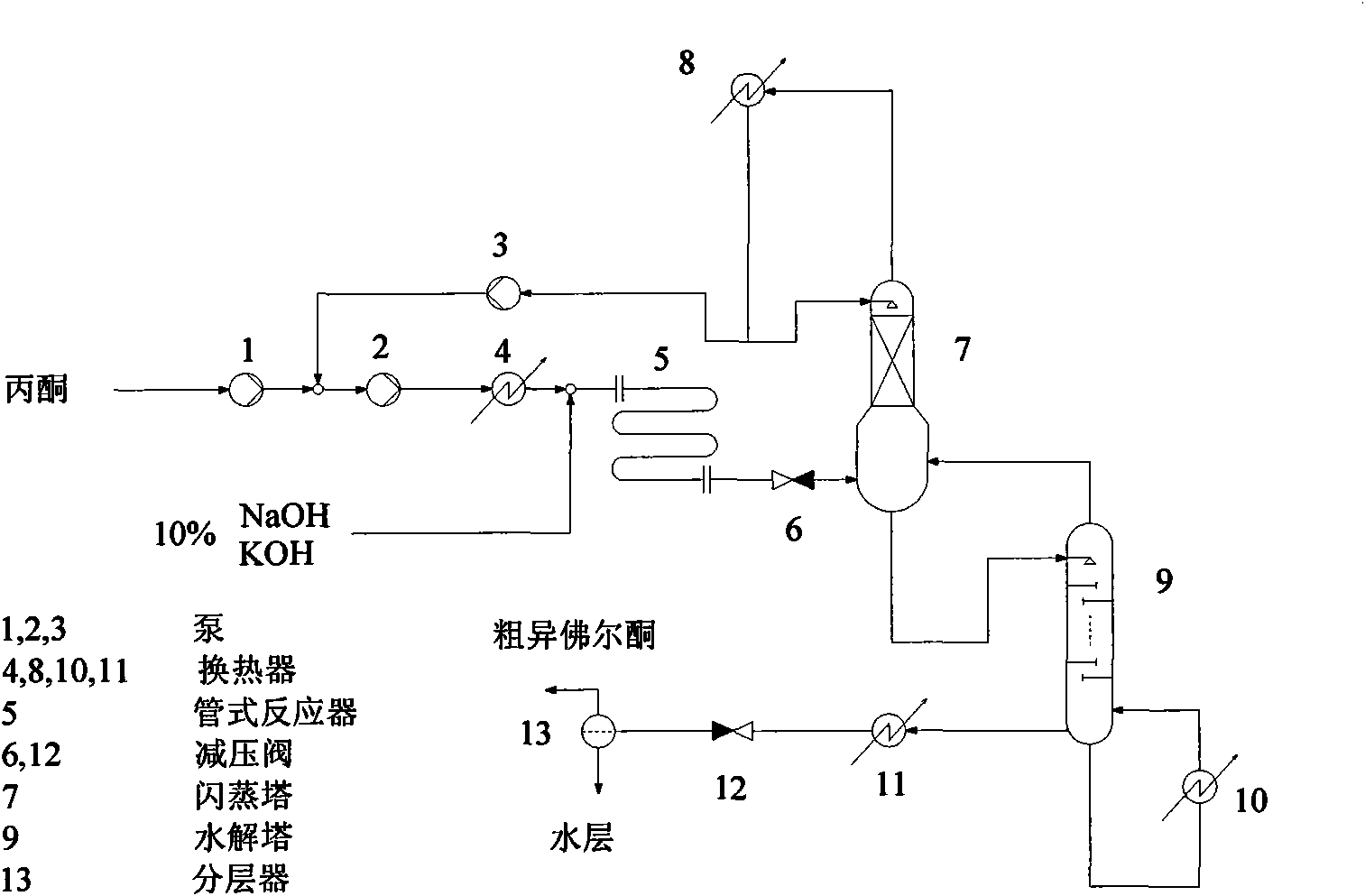
[0030] according to figure 1 In the process flow shown, acetone is pressurized to 3.2MPa through pump 1 at a flow rate of 100Kg / hour under continuous steady-state operating conditions, and then mixed with the recovered acetone mixture, and then pressurized to 8.0MPa through pump 2. After being preheated to 280°C by preheater 4, it is mixed with 2Kg / hour 10% NaOH solution pressurized to the same pressure, and then enters pipeline reactor 5 for supercritical reaction, and the reaction residence time is 3 minutes. The reaction liquid is decompressed to 3.0MPa through the pressure reducing valve 6 and then enters the flash tower 7 with 4m AX packing, and the mixture of unreacted acetone and intermediate mesityl oxide evaporated from the top of the tower is condensed and recovered by the condenser 8 Acetone mixture. The top reflux ratio of the flash tower is controlled to be 0.3, and the continuously recovered acetone mixture is boosted by the pump 3 and recycled for reaction. Th...
Embodiment 2
[0032] according to figure 1In the process flow shown, under continuous steady-state operating conditions, acetone is pressurized to 4.2MPa through pump 1 at a flow rate of 100Kg / hour, then mixed with the recovered acetone mixture, and then pressurized to 20.0MPa through pump 2. After being preheated to 320°C by preheater 4, it is mixed with 0.5Kg / hour 10% NaOH solution pressurized to the same pressure, and then enters pipeline reactor 5 for supercritical reaction, and the reaction residence time is 2 minutes. The reaction liquid is decompressed to 4.0 MPa by the pressure reducing valve 6 and then enters the flash tower 7 with 2 meters of CY packing, and the mixture of unreacted acetone and intermediate mesityl oxide evaporated from the top of the tower is condensed and recovered by the condenser 8 Acetone mixture. The top reflux ratio of the flash tower is controlled to be 0.3, and the continuously recovered acetone mixture is boosted by the pump 3 and recycled for reaction....
Embodiment 3
[0034] according to figure 1 In the process flow shown, acetone is pressurized to 3.7MPa through pump 1 at a flow rate of 100Kg / hour, and then mixed with the recovered acetone mixture, and then pressurized to 15.0MPa through pump 2. After being preheated to 300°C by preheater 4, it is mixed with 5Kg / hour 10% KOH solution pressurized to the same pressure, and then enters pipeline reactor 5 for supercritical reaction, and the reaction residence time is 1 minute. The reaction liquid is decompressed to 3.5 MPa by the pressure reducing valve 6 and enters the flash tower 7 with 3 meters of BX packing, and the mixture of unreacted acetone and intermediate mesityl oxide evaporated from the top of the tower is condensed and recovered by the condenser 8 Acetone mixture. The top reflux ratio of the flash tower is controlled to be 0.3, and the continuously recovered acetone mixture is boosted by the pump 3 and recycled for reaction. The liquid in the flash tower enters the hydrolysis to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com