A method for preparing materials having antithrombotic activity from muskrat musk and materials obtained from the method
An anti-thrombotic and muskrat technology, applied in the field of high-efficiency anti-thrombotic agents, anti-thrombotic agents, and the preparation of anti-thrombotic agents, to achieve the effect of increasing added value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Embodiment 1: the ethanol extract of muskrat incense
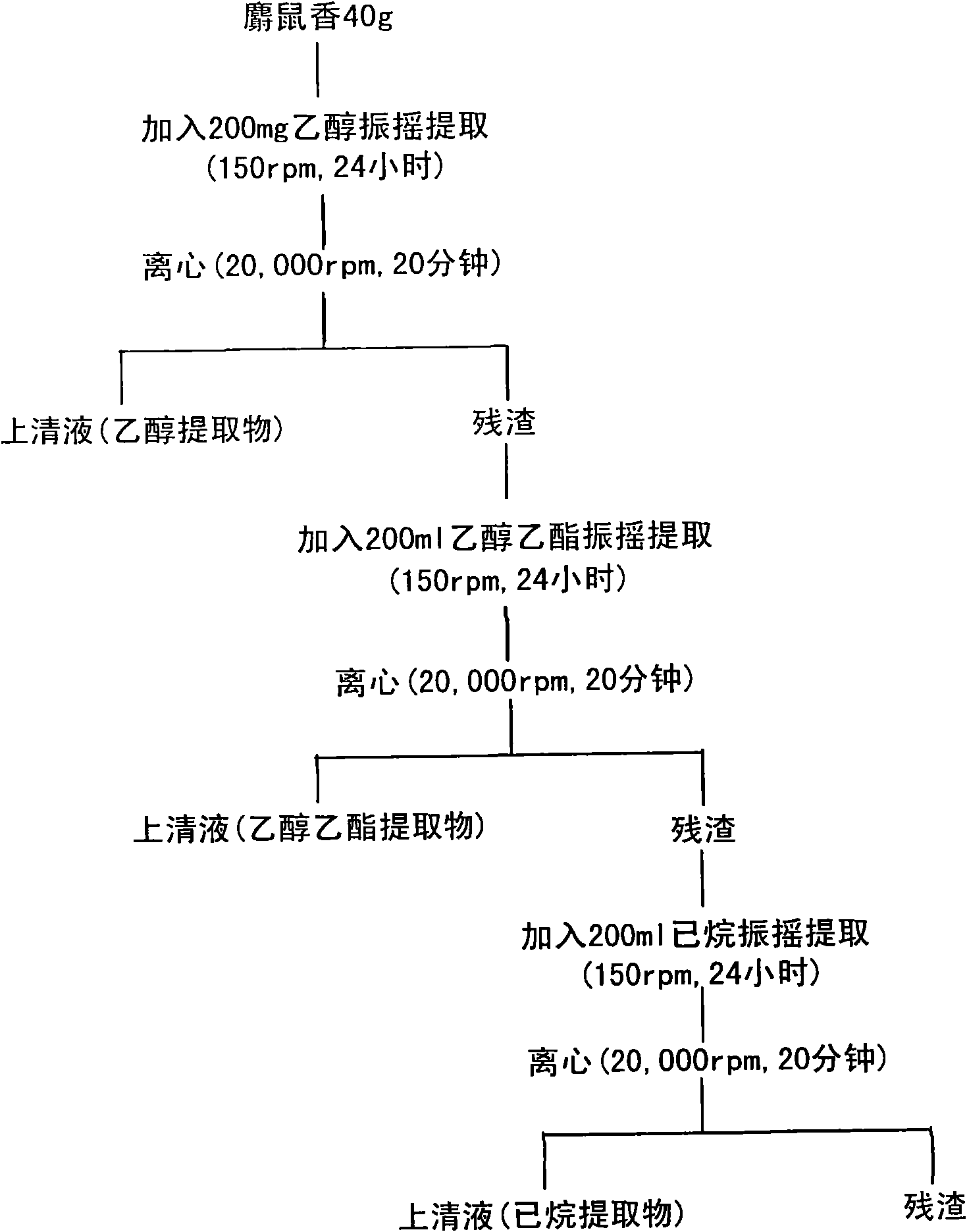
[0073] see figure 1 , muskrat incense is continuously extracted with an organic solvent, and the muskrat incense is collected from muskrats and stored in a cool room.
[0074] Specifically, 40 g of muskrat was added to an Erlenmeyer flask, ethanol (5 times the weight of muskrat, 200 ml) was added thereto, and the mixture was shaken at 150 rpm for 24 hours and centrifuged at 20,000 rpm to obtain a supernatant.
[0075] An equal amount of ethanol was added to the residue to repeat the ethanol extraction to obtain another ethanol extract other than the above ethanol extract.
[0076] Add 200ml of ethyl acetate to the residue, and collect the ethyl acetate extract according to the method shown in ethanol extraction. 200 ml of hexane was further added to the residue, and the hexane extract was collected according to the method shown in ethanol extraction.
Embodiment 2
[0078] Example 2: The 1st normal phase column chromatography and thin layer chromatography of ethanol extract of muskrat incense
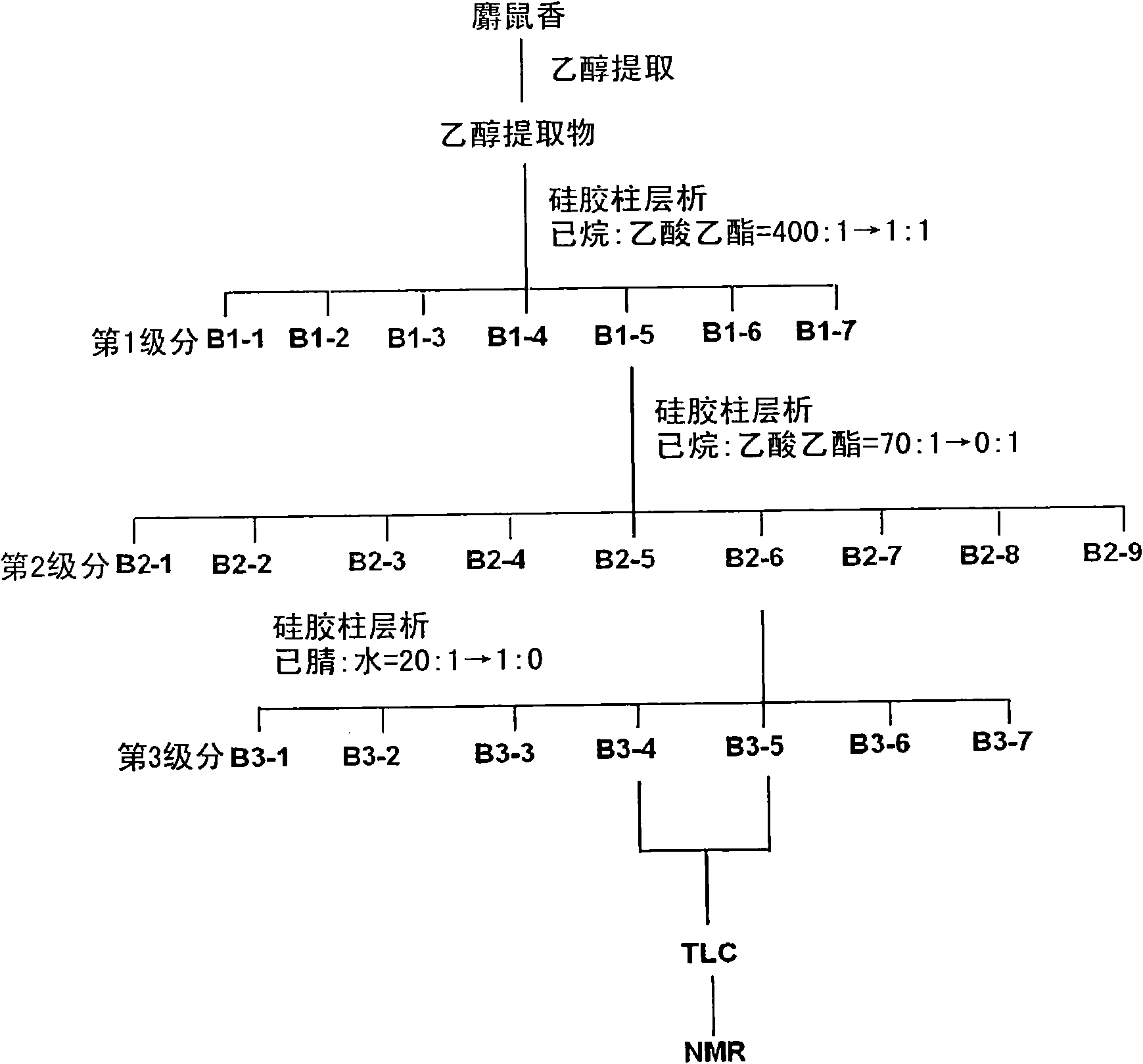
[0079] see figure 2 , the ethanol extract obtained in Example 1 was subdivided through the first normal phase column chromatography.
[0080] The column filler was normal phase silica gel (230 to 400 mesh), and the solvent as the mobile phase was hexane and ethyl acetate, wherein the combined ratio of the solvents was increased from 400:1 to 1:1, and 116 fractions were obtained.
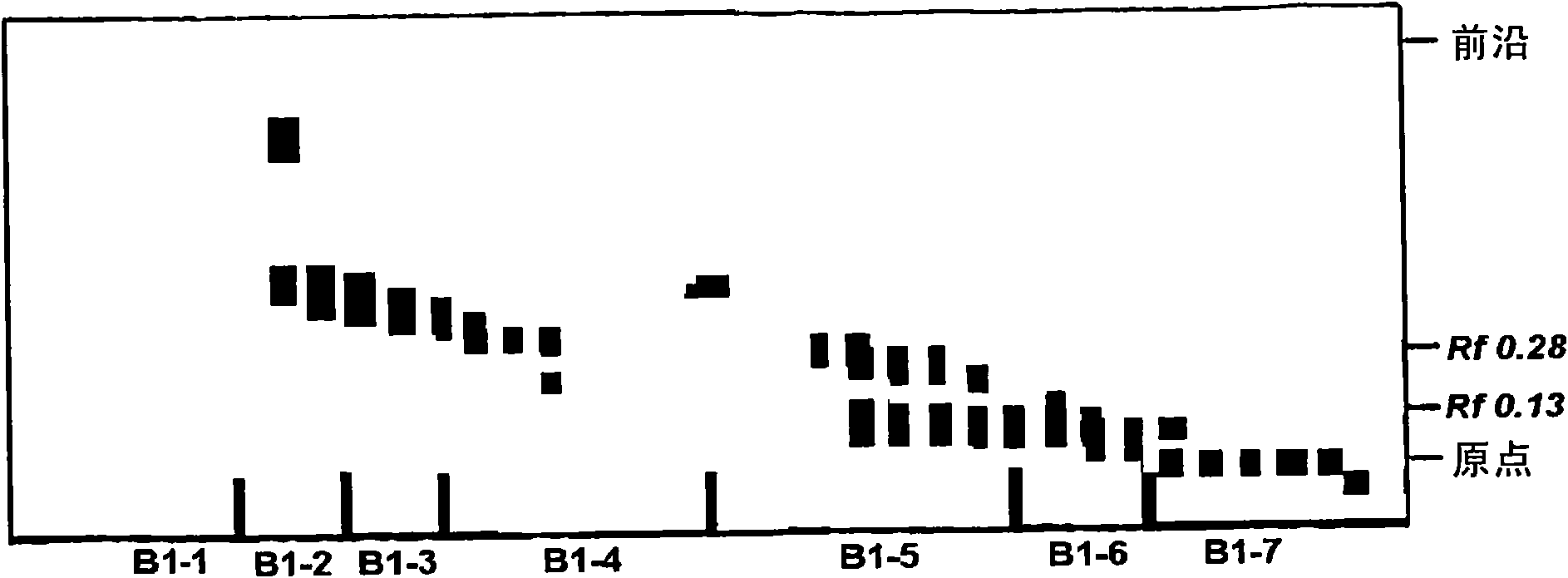
[0081] Thin-layer chromatography was used to identify the material spectrum of each fraction, and the developing solvent used was hexane and ether (10:1.5).
[0082] The spectrum of each fraction was further identified by short-wave UV lamp (254nm) and long-wave UV lamp (365nm). After treating the thin-layer chromatography plate with 10% sulfuric acid and heating, the spectrum was again identified by the colored spots.
[0083] According to the above identification ...
Embodiment 3
[0090] Example 3: The second normal phase column chromatography and thin layer chromatography of the first fraction
[0091] see figure 2 , in Example 2, the B1-5 group showed higher antithrombotic activity than aspirin, and this group was subdivided by the second normal phase column chromatography.
[0092] The solvent used as the mobile phase was hexane and ethyl acetate, wherein the combined ratio of the solvents was increased from 70:1 to 1:1, and 172 fractions were obtained.
[0093] According to the method shown in Example 2, identify the collection of collections of each fraction obtained above, and the results are listed in Figure 5 middle. Each fraction was divided into 9 groups (B2-1 to B2-9) according to the moving distance, and used for measurement of antithrombotic activity.
[0094] Measure the antithrombotic activity of the material obtained by the normal phase column for the second time according to the method in Example 2, and as Image 6 As shown, in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com