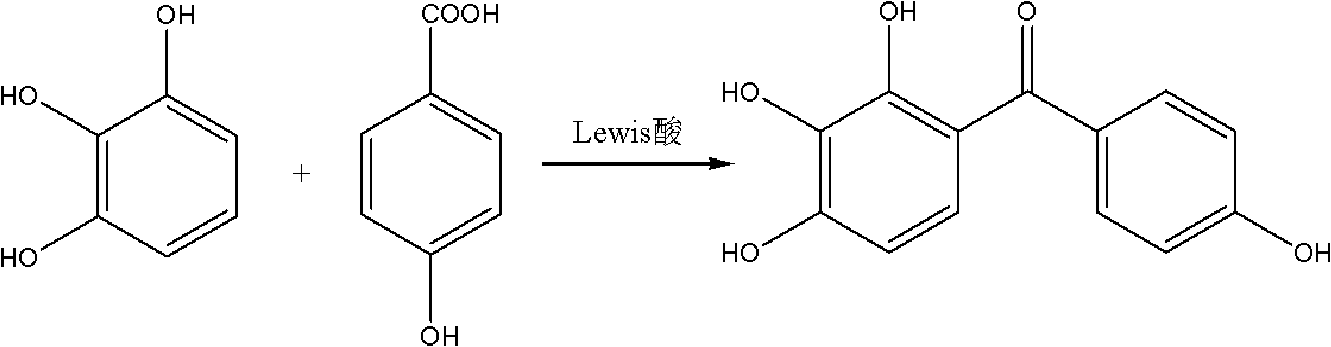
Preparation method of 2,3,4,4'-tetrahydroxyldiphenylketone
A technology of tetrahydroxybenzophenone and p-hydroxybenzoic acid is applied in the preparation of carbon-based compounds, the preparation of organic compounds, chemical instruments and methods, etc. Large-scale production, long resin regeneration time and other problems, achieve the effect of complete catalytic reaction, low production cost, mild and safe reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation of supported Lewis acid catalyst
[0028] 15.0g of purified montmorillonite was acidified in 600g of 30% sulfuric acid solution at 100°C for 4 hours, washed with deionized water and filtered, dried at 100°C for 8 hours to obtain acidified soil support, and then immersed in 21.8 g Commercially available anhydrous ZnCl 2 In the 1000mL methanol solution, after natural drying, place it in a muffle furnace and pass N at 120℃ 2 Protected roasting activation for 8 hours, after activation placed in P 2 O 5 Cool in a desiccator to obtain 32.4g of the ZnCl with a loading capacity of 6mmol / g 2 / Montmorillonite catalyst.
Embodiment 2
[0030] The impregnation solution contains 21g of commercially available anhydrous AlCl 3 1000mL methanol solution, the calcination temperature is 100℃, and other conditions are the same as in Example 1, 31.5g of the AlCl with a loading amount of 6mmol / g can be obtained 3 / Montmorillonite catalyst.
[0031] Implementation column 3
[0032] 25.0g of purified montmorillonite was acidified in 1200g of 30% sulfuric acid solution at 100°C for 4 hours, washed with deionized water and filtered, dried at 100°C for 8 hours to obtain acidified soil support, and then immersed in 35g Commercially available anhydrous ZnBr 2 In 1000mL methanol solution, after natural drying, place it in a muffle furnace and pass N at 200℃ 2 Protected roasting activation for 8 hours, after activation placed in P 2 O 5 Cool in a desiccator to obtain 54.0 g of the ZnBr with a loading capacity of 6 mmol / g 2 / Montmorillonite catalyst.
Embodiment 4
[0034] As in Example 3, the preparation of the acidified soil support was the same, and the acidified soil support was immersed in 28 g of commercially available anhydrous ZnBr 2 In 1000mL methanol solution, after natural drying, place it in a muffle furnace and pass N at 200℃ 2 Protected roasting activation for 8 hours, after activation placed in P 2 O 5 Cool in a desiccator to obtain 46.0 g of the ZnBr with a loading capacity of 4 mmol / g 2 / Montmorillonite catalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com