Synthesis method of cefradine
A synthesis method and technology of cefradine, applied in the field of drug synthesis, can solve problems such as affecting the crystal form and product stability of cefradine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1. Condensation
[0025] Add 75g of dihydrophenylglycine sodium salt, 400ML of dichloromethane, 20ML of DMA, and 0.1ML of 4-picoline into a 1000ML three-necked bottle; cool down to -20~-25°C, and add 49.0g of carbonyldiimidazole in portions , the reaction is exothermic, the speed of addition should not be too fast, and the temperature should not be higher than -20°C. The reaction was stirred at ℃ for 120 minutes, and the condensation reaction was completed.
[0026] 2. Hydrolysis and decolorization
[0027] Pour the condensation solution into a 2000ML beaker, start stirring, add 300ML of purified water, DMA32ML into the 2000ML beaker, stir evenly, control the temperature at 29-33°C, adjust the pH value to 0.6-0.8 with concentrated hydrochloric acid, then add the hydrolyzate In the separating funnel, let it rest fully, remove the dichloromethane phase, collect the water phase into a 500ML beaker, add an appropriate amount of activated carbon, stir for 10 minutes, and f...
Embodiment 2
[0033] Add 70g of dihydrophenylglycine sodium salt, 400ML of dichloromethane, 25ML of DMF, and 0.2ML of 4-picoline into a 1000ML three-necked bottle; cool down to -20~-25℃, and add 49.8g of carbonyldiimidazole in portions , the reaction is exothermic, the speed of addition should not be too fast, and the temperature should not be higher than -15°C. The reaction was stirred at ℃ for 120 minutes, and the condensation reaction was completed.
[0034] Post-reaction treatment such as crystallization, steps such as washing and drying are the same as in Example 1.
Embodiment 3
[0036] Add 82g of dihydrophenylglycine sodium salt, 400ML of chloroform, 30ML of DMA, and 0.3ML of 4-picoline into a 1000ML three-necked flask; cool down to -20~-25°C, add 63.3g of carbonyldiimidazole in portions, and react Exothermic, the speed of addition should not be too fast, the temperature should not be higher than -15°C, after the dropwise addition, control the temperature at -25~-30°C, keep it for 45 minutes, then add 50g of 7-ADCA, at -20~-25°C The reaction was stirred for 120 minutes, and the condensation reaction was completed.
[0037] Post-reaction treatment such as crystallization, steps such as washing and drying are the same as in Example 1.
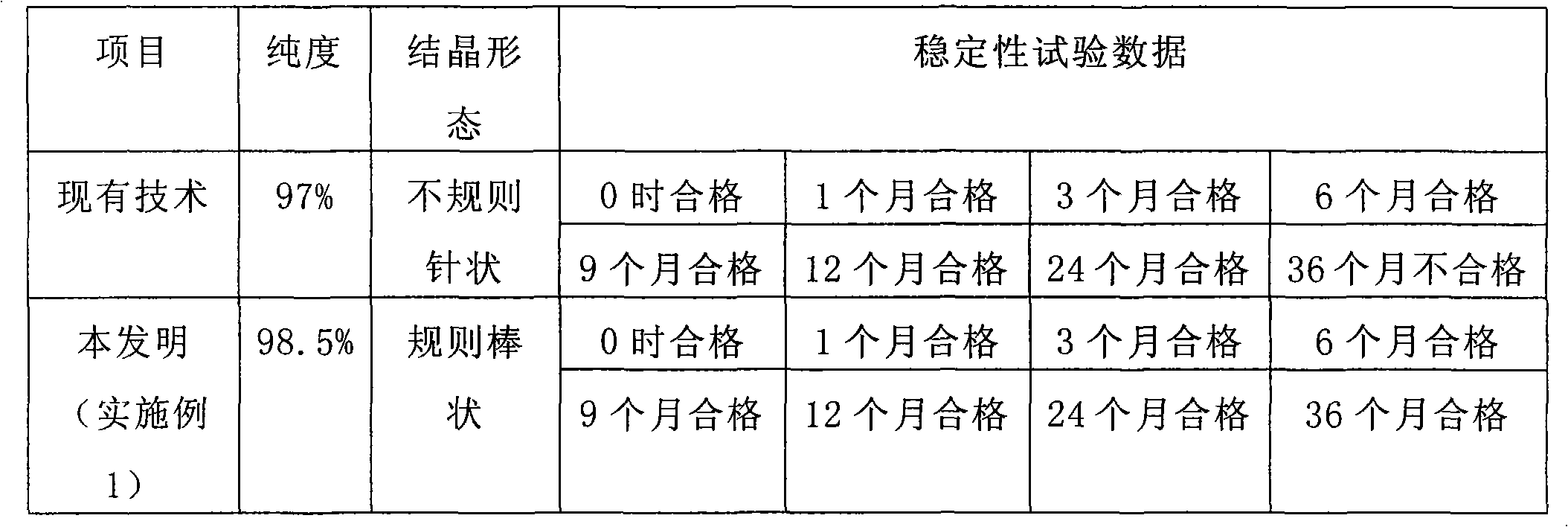
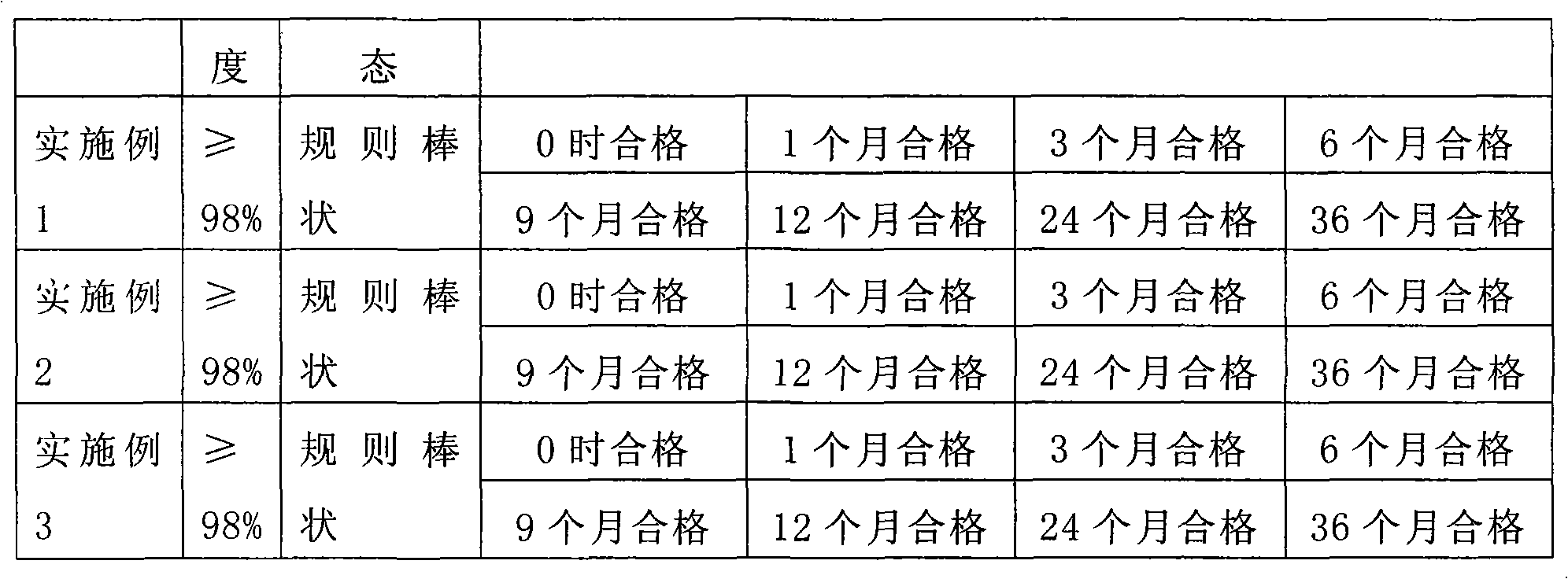
[0038] The comparison of technical quality indicators of the above implementation schemes is shown in the table below:
[0039]
[0040]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com