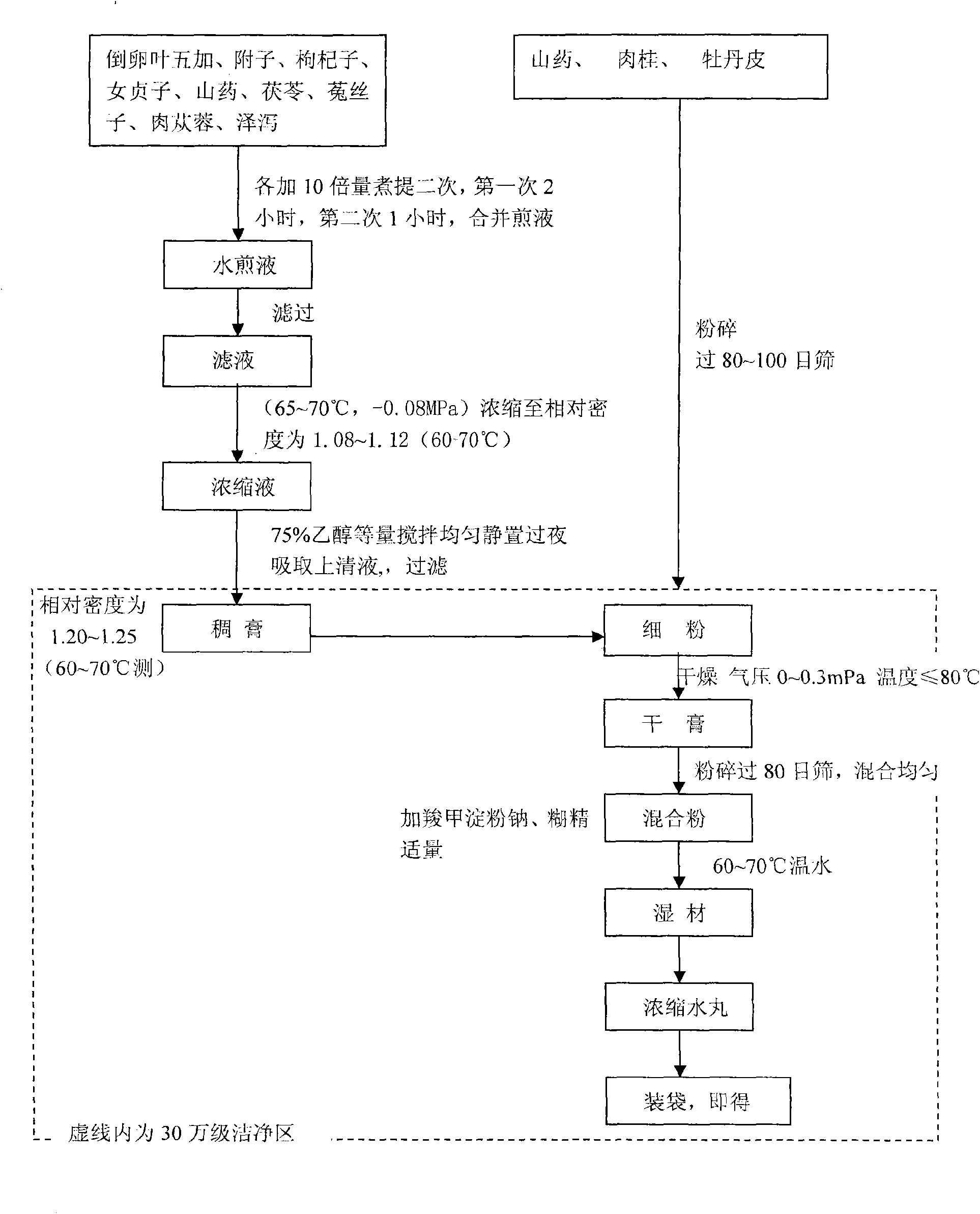
Medicament for treating climacteric syndrome and preparation method thereof
A syndrome and medicine technology, applied to medicines for the treatment of climacteric syndrome, in the field of preparation of the above-mentioned medicines, can solve problems such as treatment difficulties, impact on personal health and quality of life, etc., and achieves reduction of production costs, production equipment and technical requirements are simple, Easy to swallow effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0023] Study on the crushing yield and sterilization process of powdered medicinal materials
[0024] 1.1 Investigation on the crushing yield of powdered medicinal materials
[0025] The original preparation of this product is to pulverize the powdered medicinal materials. For the convenience of control, the medicinal materials are provisionally crushed and passed through a 100-mesh sieve, and the crushing yield of the powdered medicinal materials is investigated.
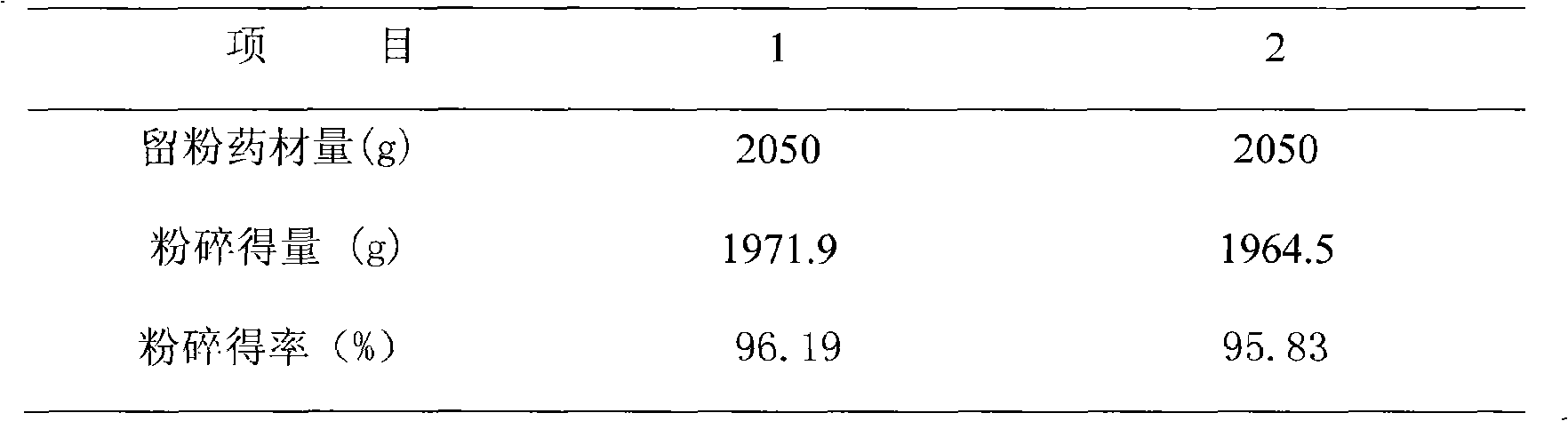
[0026] Test method: Weigh 1000g of dried Chinese yam, 350g of cinnamon and 700g of Moutan cortex, a total of 2050g of medicinal materials, 2 parts each, mix them separately, crush them, pass through a 100-mesh sieve, collect the fine powders respectively, weigh them, and calculate the powder yield. The results are shown in Table 1.
[0027] Table 1 Investigation of crushing yield
[0028]
[0029] The results show that the pulverization yield of the powdered medicinal material of this product is about 96%.
...
experiment example 2
[0032] Experimental example 2: Extraction process research
[0033] 2.1 Pre-test
[0034] According to the proportion of the prescription, accurately weigh 65g of Acanthopanax ovalica, 100g of rehmannia glutinosa, 45g of aconite (manufactured), 65g of medlar, 50g of Ligustrum lucidum (manufactured), 35g of Poria, 65g of dodder (manufactured), and 65g of Cistanche (made) 35g of Alisma, add 10 times the amount of water and decoct for 2 hours, combine the decoction, filter, and calculate the liquid absorption.
[0035] Conclusion: the amount of liquid absorbed by medicinal dregs is 10 times the amount of water 5250ml- filtrate 4000ml = the amount of liquid absorbed is 1250ml, and the amount of liquid absorbed by medicinal materials is 2.4 times.
[0036] 2.2 Test
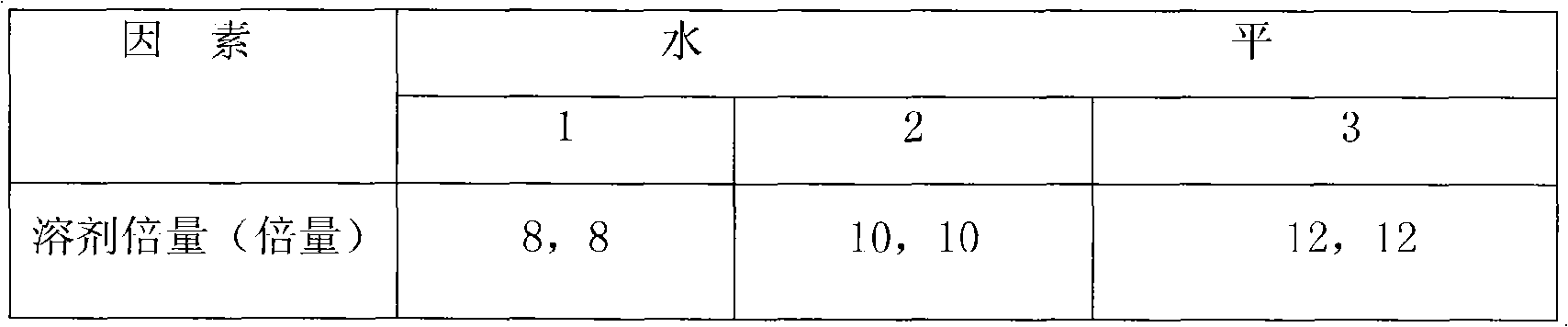
[0037] 2.2.1 Optimizing the amount of water added due to the fact that the original water extraction process has formulated more detailed and reasonable parameters for the main factors such as the extraction time and...
experiment example 3
[0060] Experimental example 3: Concentration process research
[0061] 3.1 Liquid Concentration Method
[0062] At present, most of the industrial production uses decompression equipment for concentration. This method not only has high efficiency, but also helps to retain active ingredients. Therefore, after extracting the medicinal liquid and recovering ethanol, concentration under reduced pressure is adopted. Through experiments, it is determined that the concentration condition is concentration under reduced pressure (65-70°C, -0.08MPa). The test shows that the method has high efficiency and no bumping, so this condition is determined.
[0063] 3.2 Conditions for recovering ethanol
[0064] Ethanol recovery under reduced pressure has high efficiency and is suitable for large-scale industrial production. The present invention adopts the method of recovering ethanol under reduced pressure and investigates its specific process parameters. Combined with experience and experim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com