Catalyst for greenly synthesizing halogenated arylamine by means of high-efficiency catalytic hydrogenation of halogenated aromatic nitro compound and preparation method thereof
An aromatic nitro group and catalytic hydrogenation technology, which is applied in the field of catalysts for the catalytic hydrogenation of halogenated aromatic nitro compounds to synthesize halogenated aromatic amines, can solve problems such as high activity, and achieve good performance, high raw material utilization, and stability. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
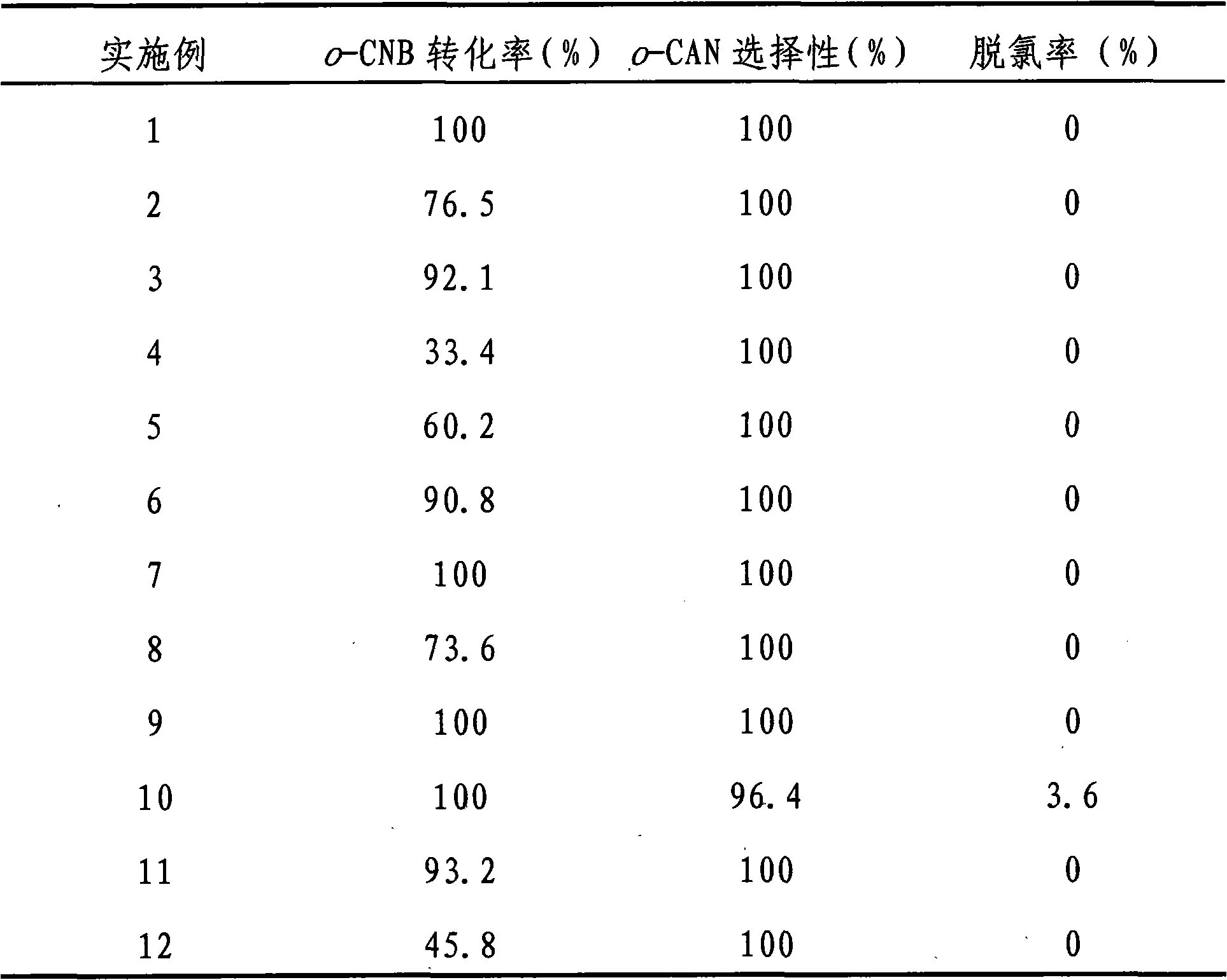
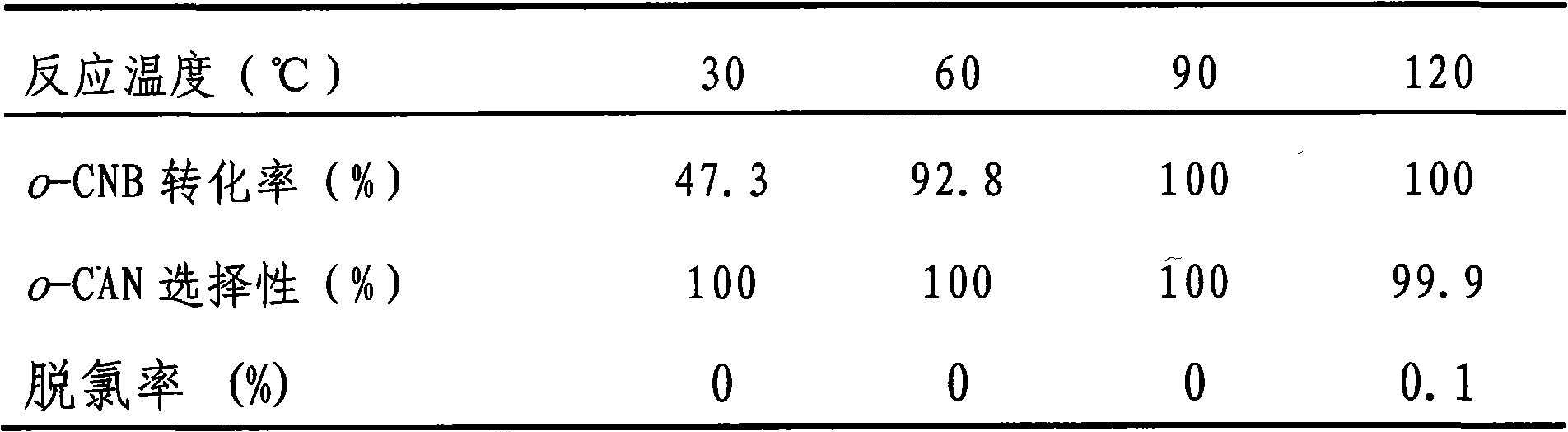
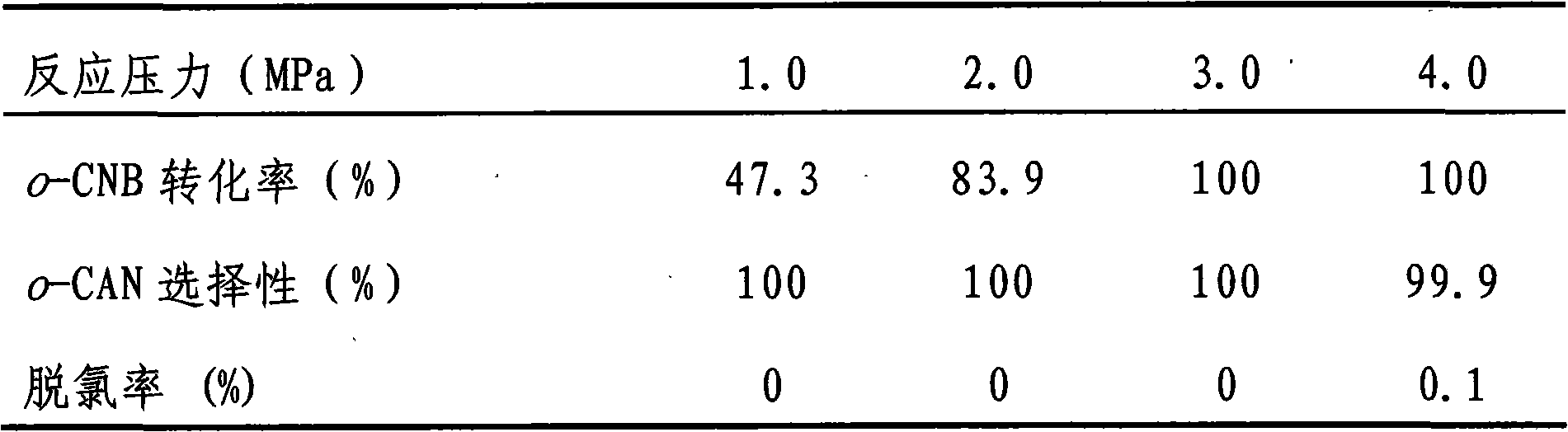
Embodiment 1
[0018] Preparation of TiO by urea deposition-precipitation method 2 Supported Au and Pt bimetallic catalysts. According to the weight ratio of metal to carrier and two metal elements Au and Pt, 2.5×10 -5 mol / L HAuCl 4 And 1×10 -5 mol / L H 2 PtCl 6 Aqueous solution, add 2.0mL HAuCl 4 Aqueous solution (2.5×10 -5 mol / L) and 0.5mL H 2 PtCl 6 Aqueous solution (1×10 -5 mol / L) was added to 47.5mL of distilled water, 2 grams of carrier and 1.5 grams of urea were added to it, and the temperature was raised to 85°C under constant stirring and kept at this temperature for 2 to 5 hours. In this example, 4 hours were used, at room temperature Aging for 5-10 hours, 5 hours in this embodiment, washing with deionized water until no chlorine ions, drying at 50-110°C, drying at 110°C and roasting at 200-500°C for 2-5 hours in this embodiment, In this embodiment, calcination at 200°C for 5 hours is used to obtain a supported catalyst with an Au loading of 0.5 wt% and a Pt loading of 0.05 wt%.
Embodiment 2
[0020] Preparation of TiO by ammonia precipitation-precipitation method 2 Supported Au and Pt bimetallic catalyst. According to the weight ratio of metal to carrier and two metal elements Au and Pt, 2.5×10 -5 mol / L HAuCl 4 And 1×10 -5 mol / L H 2 PtCl 6 Aqueous solution, add 2.0mLHAuCl 4 Aqueous solution (2.5×10 -5 mol / L) and 0.5mL H 2 PtCl 6 Aqueous solution (1×10 -5 mol / L) was added to 47.5mL of distilled water, under constant stirring, 0.3% (w) ammonia was added dropwise to adjust the pH to 9.0, then 2 g of carrier was added, and ammonia was added dropwise to re-adjust the pH to 9.0, stirring for 5 hours , Aging at room temperature for 10 hours, washing with deionized water until there is no chloride ion, washing, drying at 110°C, and calcining at 200°C for 5 hours to obtain 0.5wt% Au loading and 0.05wt% Pt loading Supported catalyst.
Embodiment 3
[0022] Preparation of ZrO by urea deposition-precipitation method 2 Supported Au and Pt bimetallic catalysts. The preparation steps are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com