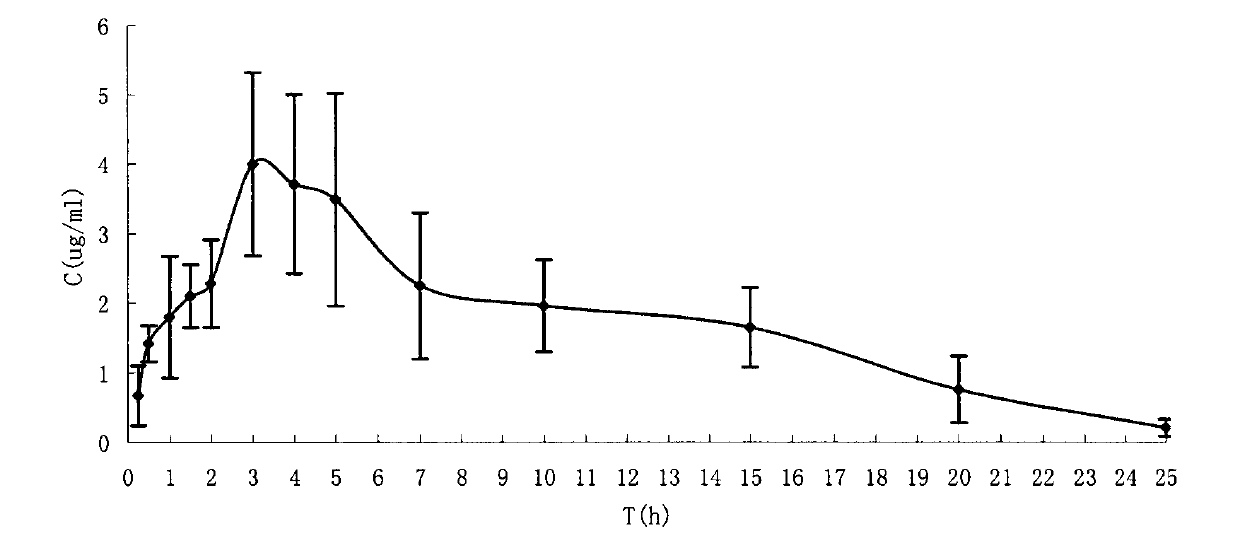
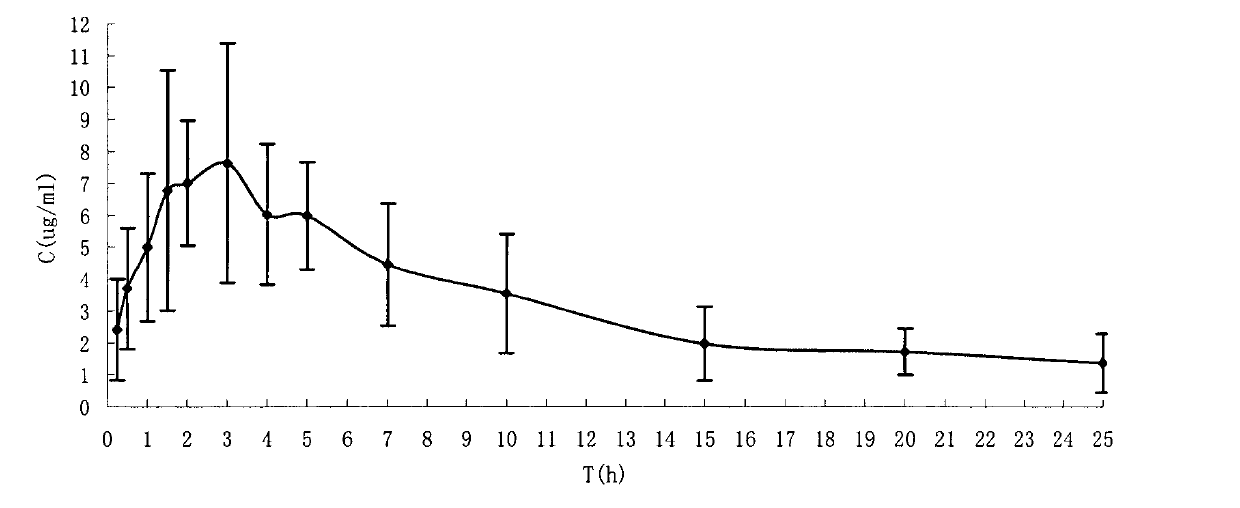
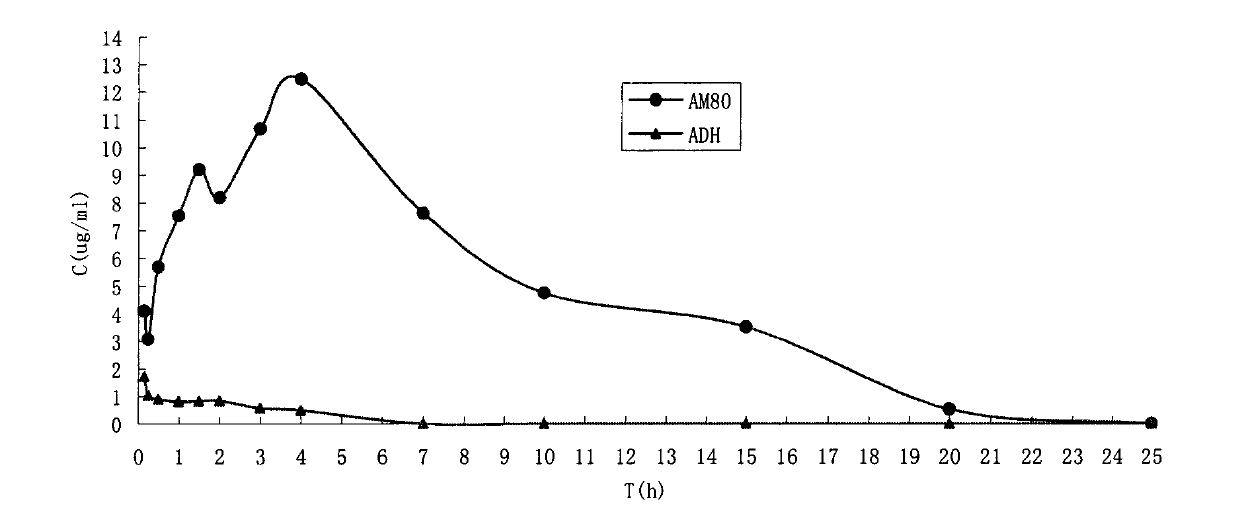
Water-soluble prodrug of tamibarotene, and preparation method and applications thereof
A tamibarotene, water-soluble technology, applied in the fields of organic compound synthesis and medical application, can solve problems such as poor water solubility, inability to delay drug release, etc., and achieve the effect of good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1: Tamibarotene N, the preparation of N-dimethylethanolamine (DMEA) ester (carbodiimide method)
[0068] Tamibarotene (3.51g, 10mmol) was dissolved in 50ml of tetrahydrofuran, then DCC (3.09g, 15mmol) and HOBt (2.03g, 15mmol) were added, and the reaction was stirred at 0°C for 12 hours to generate Tamibar 1-Hydroxybenzotriazole active ester of rotin. Then DMEA (4.46g, 50mmol) was added, and the reaction was stirred at 0°C for 24h. The reaction solution was evaporated under reduced pressure to remove the solvent tetrahydrofuran (THF). After the residue was dissolved in a small amount of ethyl acetate, silica gel column chromatography was performed. The ester was used as the eluent for elution, and the eluent was rotary evaporated under reduced pressure to remove the solvent, and the DMEA ester of tamibarotene was prepared (white powder, yield 46.3%). mp 138.0-139.0°C. ESI-MS (m / z): 423.5 (M+H). 1 HNMR (DMSO-d 6 )δ: 1.238~1.251(m, 12H, 4×CH 3 ); 1.647(s, 4...
Embodiment 2
[0069] Embodiment 2: Tamibarotene N, the preparation of N-dimethylethanolamine (DMEA) ester (CDI method)
[0070] Tamibarotene (3.51 g, 10 mmol) was dissolved in 50 ml of tetrahydrofuran, then CDI (4.86 g, 30 mmol) was added, and the reaction was stirred at room temperature for 24 hours. Then DMEA (4.46g, 50mmol) was added, and the reaction was stirred at room temperature for 24 hours. The reaction solution was evaporated under reduced pressure to remove the solvent THF, and the residue was dissolved with a small amount of ethyl acetate. The solvent was eluted, and the eluent was evaporated under reduced pressure to remove the solvent, and DMEA ester of tamibarotene (white powder, yield 75.9%) was prepared. mp 138.0-139.0°C.
Embodiment 3
[0071] Embodiment 3: Tamibarotene N, the preparation of N-dimethylethanolamine (DMEA) ester hydrochloride
[0072] Dissolve the DMEA ester of tamibarotene (4.23g, 10mmol) in 100ml of ethyl acetate, add 100ml of anhydrous HCl saturated ethyl acetate solution under stirring at room temperature, stir at room temperature for 1 hour, evaporate the solvent under reduced pressure, and then Add 200 ml of ethyl acetate to dissolve the residue, and evaporate the solvent under reduced pressure to obtain tamibarotene DMEA ester hydrochloride (white powder, yield 95.6%). mp 215.0-217.0°C. 1 HNMR (DMSO-d 6 )δ: 1.240~1.252(m, 12H, 4×CH 3 ); 1.636(s, 4H, 2×CH 2 ); 2.882(s, 6H, 2×CH 3 ); 3.546 (s, 2H, N-CH 2 ); 4.629 ~ 4.645 (t, J = 4.8Hz, 2H, O-CH 2 ); 7.293~7.308(d, J=9.0Hz, 1H, ArH); 7.575~7.589(m, 1H, ArH); 7.694~7.697(d, J=1.8Hz, 1H, ArH); 8.093~8.107(d , J=8.4Hz, 2H, 2×ArH); 8.194~8.208 (d, J=8.4Hz, 2H, 2×ArH); 10.114 (s, 1H, NH); 10.320 (s, 1H, HCl).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com