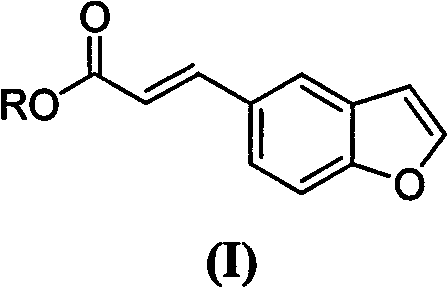
(E)-3-(benzfuran-5-yl) acrylic ester compound, preparation method and application thereof
A technology of acrylates and benzofuran, applied in the field of preparation of 3-propionic acid, can solve the problems of "three wastes" discharge, harsh reaction conditions, cumbersome reaction operation and post-treatment process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
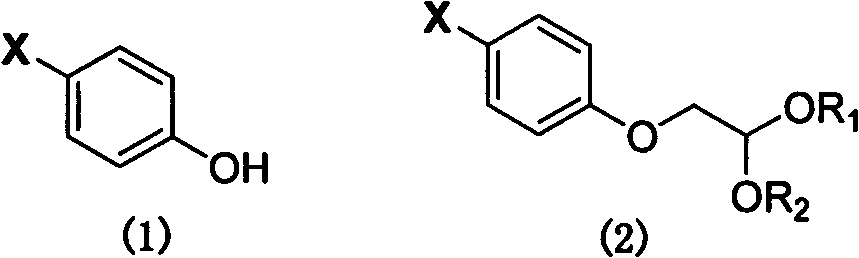
[0044] Preparation of 2-(4-bromophenoxy)-diethylacetal (2a)
[0045] Add 50.0g (0.29mol) of p-bromophenol, 62.6g (0.32mol) of bromoacetal, anhydrous K 2 CO 3 48.03g (0.35mol) and 500ml of acetone, heated and refluxed and stirred for 18h. After the reaction, filtered, the filtrate was evaporated to remove the solvent under reduced pressure, and the residual oil was dissolved in 500ml of chloroform. NaCl aqueous solution 100ml washed, the organic layer was washed with anhydrous NaCl 2 SO 4 Dry, filter, and evaporate the solvent under reduced pressure to obtain 77.15 g of 2-(4-bromophenoxy)-diethyl acetal, with a yield of 92.0%.
Embodiment 2
[0047] Preparation of 2-(4-bromophenoxy)-acetaldehyde (2b)
[0048]The operation process is the same as in Example 1, except that bromoacetal is replaced by 40% aqueous solution of chloroacetaldehyde, and acetone is replaced by N,N-dimethylformamide to obtain 2-(4-bromophenoxy)-ethyl Aldehyde, yield 83.9%.
Embodiment 3
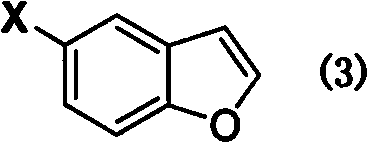
[0050] Preparation of 5-bromobenzofuran (3)
[0051] Add 28.92g (0.1mol) of 2-(4-bromophenoxy)-diethyl acetal, 30.0g polyphosphoric acid, and 200ml 1,2-dichloroethane into the reaction flask, heat up and reflux and stir for 5h, After the reaction is over, add 100ml of ice water, stir evenly, separate the organic layer, wash with 10% aqueous sodium carbonate solution 50ml, saturated NaCl aqueous solution 25ml successively, and wash the organic layer with anhydrous NaCl 2 SO 4 Dry, filter, and distill under reduced pressure to obtain 16.82 g of light yellow 5-bromobenzofuran liquid, with a yield of 85.3%. 1 H NMR (CDCl 3 , 400MHz) δ: 7.75 (s, 1H, Ar-H 4 ), 7.64 (d, J=2.0Hz, 1H, Ar-H 2 ), 7.41 (d, J=8.8Hz, 1H, Ar-H 6 ), 7.39 (d, J=8.8Hz, 1H, Ar-H 7 ), 6.74 (d, J=2.0Hz, 1H, Ar-H 3 ); 13 C NMR (CDCl 3 , 100MHz) δ: 153.6 (Ar-C 7a ), 146.2 (Ar-C 2 ), 129.2 (Ar-C 3a ), 127.0 (Ar-C 6 ), 123.8 (Ar-C 4 ), 115.6 (Ar-C 5 ), 112.8 (Ar-C 7 ), 106.2 (Ar-C 3 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com