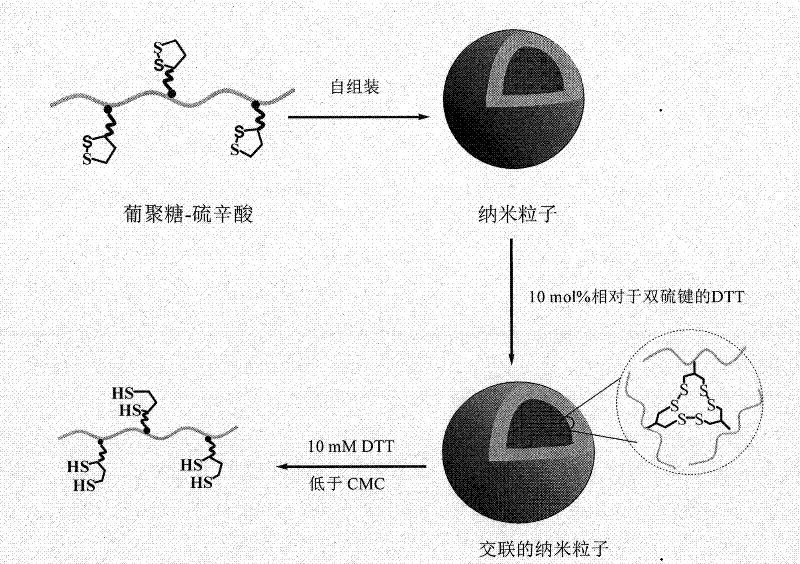
Hydrophilic polymer the side chain of which is modified by lipoic acid and preparation and application thereof
A hydrophilic polymer and amphiphilic polymer technology, which is applied in the directions of non-active ingredients, such as medical preparations, educts, pharmaceutical formulations, etc. Efficiency, overcoming the effect of being easily leaked
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
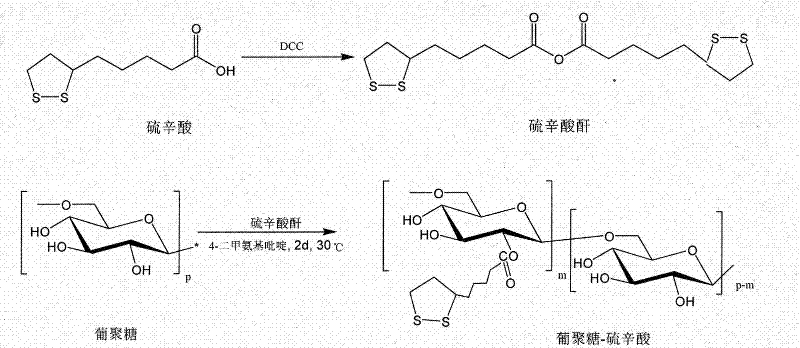
[0038] Embodiment one, synthetic polymer Dex-LA (M n dextran =20kDa, DS=80%)
[0039] Under argon protection, lipoic acid (0.639g, 3.10mmol) was dissolved in 10mL of dichloromethane, added to a 50mL Schlenk vacuum-sealed bottle, and DCC dissolved in 5.0mL of dichloromethane was (0.384g, 1.86mmol) into a sealed bottle, put the bottle in an oil bath at 30°C, stir and react for 22 hours, cool, filter to remove the urea generated in the reaction, spin the filtrate, and remove the solvent to obtain lipoic anhydride .
[0040] The lipoic anhydride obtained above was added to 3 mL of anhydrous-treated dimethyl sulfoxide. In a 50mL three-necked flask, add dextran (0.25g, 1.55mmol AHG) dissolved in 19mL of dimethyl sulfoxide, and then add lipoic anhydride and dextran dissolved in 2mL of dimethyl sulfoxide in sequence under argon protection. 4-Dimethylaminopyridine (0.189g, 1.55mmol), the reactor was placed in an oil bath at 30°C, stirred and reacted for 48 hours, then precipitated i...
Embodiment 2
[0041] Embodiment two, synthetic polymer Dex-LA (M n dextran =20kDa, DS=20%)
[0042] Under argon protection, lipoic acid (0.255g, 1.24mmol) was dissolved in 10mL of dichloromethane, added to a 50mL Schlenk vacuum-sealed bottle, and DCC dissolved in 5.0mL of dichloromethane was dissolved under argon. (0.154g, 0.74mmol) into a sealed bottle, put the bottle in an oil bath at 30°C, stir and react for 22 hours, cool, filter to remove the urea generated in the reaction, spin the filtrate, and remove the solvent to obtain lipoic anhydride .
[0043] The lipoic anhydride obtained above was added to 3 mL of anhydrous-treated dimethyl sulfoxide. In a 50mL three-necked flask, add Dextran (0.25g, 1.55mmol AHG) dissolved in 19mL dimethyl sulfoxide, and then add lipoic anhydride and 4-dimethylamino dissolved in 2mL dimethyl sulfoxide in sequence under argon protection. Pyridine (0.076g, 0.62mmol), the reactor was placed in an oil bath at 30°C, stirred and reacted for 48 hours, then prec...
Embodiment 3
[0044] Embodiment three, synthetic polymer Dex-LA (M n dextran =70kDa, DS=40%)
[0045] Under argon protection, lipoic acid (0.352g, 1.71mmol) was dissolved in 10mL of dichloromethane, added to a 50mL Schlenk vacuum-sealed bottle, and DCC dissolved in 5.0mL of dichloromethane was (0.212g, 1.03mmol) into a sealed bottle, put the bottle in an oil bath at 30°C, stir and react for 22 hours, cool, filter to remove the urea generated in the reaction, spin the filtrate to obtain lipoic anhydride after removing the solvent .
[0046] The lipoic anhydride obtained above was added to 3 mL of anhydrous-treated dimethyl sulfoxide. In a 50mL three-necked flask, add Dextran (0.25g, 1.55mmol AHG) dissolved in 19mL dimethyl sulfoxide, and then add lipoic anhydride and 4-dimethylamino dissolved in 2mL dimethyl sulfoxide in sequence under argon protection. Pyridine (0.104g, 0.86mmol), the reactor was placed in an oil bath at 30°C, and after stirring and reacting for 48 hours, it was precipit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com