A kind of hydromethylation reaction catalyst and method for preparing isononanoic acid
A catalyst, chemical reaction technology, applied in the direction of carbon monoxide or formate reaction preparation, physical/chemical process catalyst, organic compound/hydride/coordination complex catalyst, etc., can solve the problem of poor hydroformylation reaction activity and selectivity , low activity and selectivity, etc., to achieve the effect of complex stability, improved catalytic activity, and strong binding ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation of embodiment 1 ligand
[0041] a) Preparation of Ligand 1:
[0042] 83.6g carbazole ( Add 0.5mol) of the compound into 500ml of n-hexane solvent and mix evenly, heat to 65°C, then dropwise add 1000ml of n-hexane solvent containing 41.2g of phosphorus trichloride (0.3mol) to the system, and stir for 100min to obtain ligand 1 The solution; the solvent is recovered by distillation, and then dried to obtain a white blocky solid, which is recrystallized with ethyl acetate to obtain a white powdery ligand 1 product with a structure of The nuclear magnetic analysis data are as follows: 1 H NMR (300MHz, CDCL3): δ=7.25-7.33(m,9H), 7.50(m,3H), 7.63(m,3H), 7.94(m,3H), 8.12(m,3H), 8.55(m ,3H), 13 C-NMR (300MHz, CDCl3): δ=129.7, 121.7, 121.4, 119.8, 115.7, 111.1.
[0043] b) Preparation of Ligand 2:
[0044] 60g purine ( Add 0.5mol) of the compound into 500ml of n-hexane solvent and mix evenly, heat to 65°C, then dropwise add 1000ml of n-hexane solvent cont...
Embodiment 2 2
[0049] Embodiment 2 diisobutylene hydrogen methyl esterification reaction:
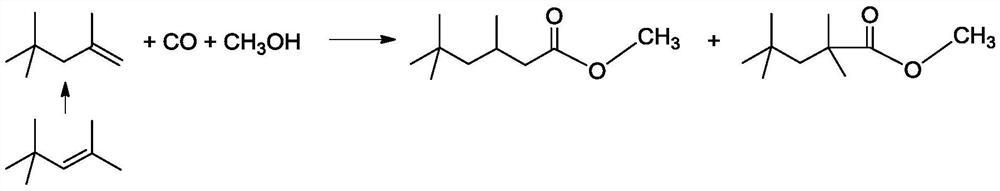
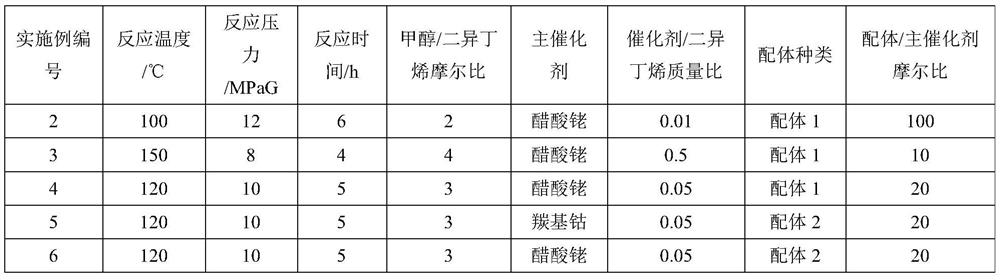
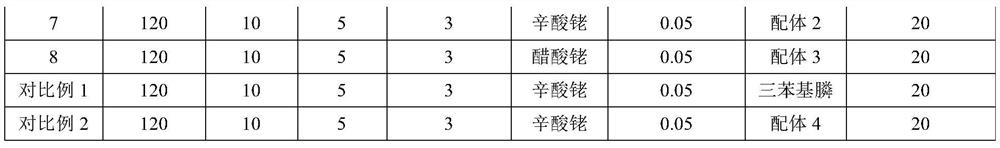
[0050] Add 500g diisobutene (4.46mol), 286g methanol (8.93mol), 0.13g (rhodium relative to diisobutene mass fraction is 0.01%) rhodium acetate (Rh content: ≥ 39.0wt%), West Asia reagent ) and 26.3g of Ligand 1 were mixed evenly to obtain a reaction liquid; after the reaction liquid in the reactor was heated up to 100°C, the pressure of carbon monoxide gas was introduced to 12MPa, and the reaction was carried out at constant temperature and pressure for 6 hours. , using Agilent-7820 gas chromatography to analyze the composition of the reaction solution, the results show that the conversion rate of diisobutene is 95.6%, the selectivity of methyl isononanoate is 97.5%, and the methyl isononanoate is 3,5,5-trimethylhexanoate 99.6%. The above reaction solution was distilled to remove unreacted methanol and diisobutylene at the top of the tower, and then 714 g of crude methyl isononanoate was obtained at t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com