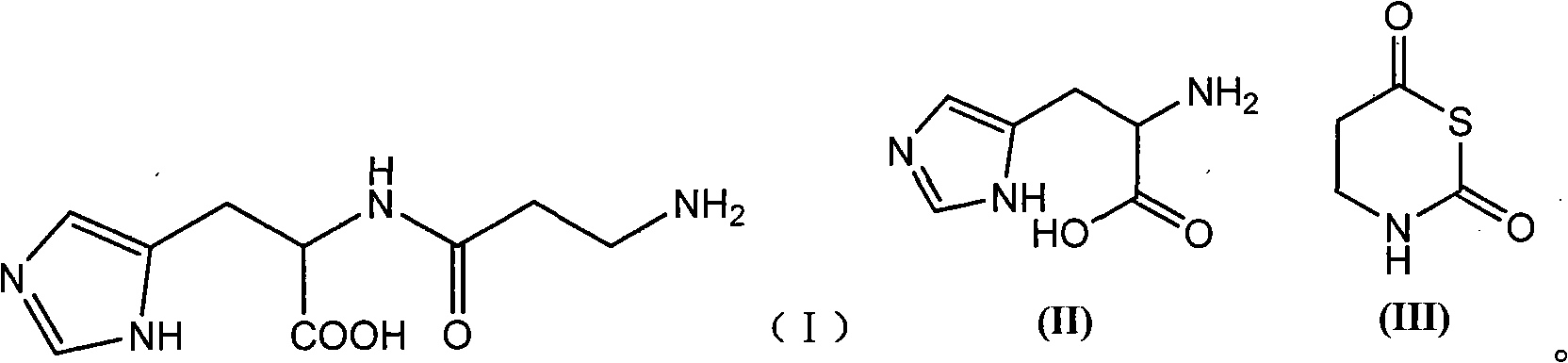
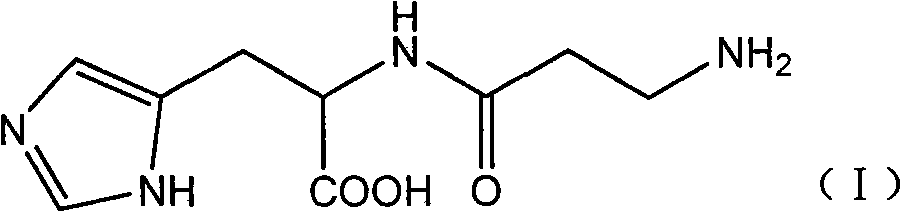
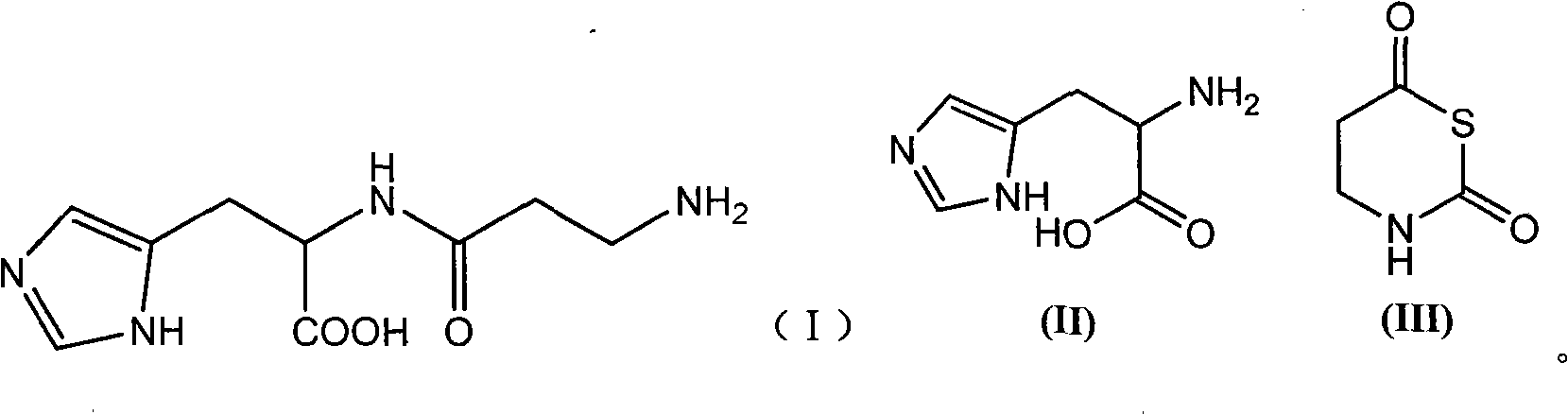
Preparation method of L-carnosine
A carnosine and histidine technology, applied in organic chemistry and other directions, can solve problems such as impact on application, pollution, low total yield, etc., and achieve the effects of being beneficial to industrial production, simple process, and low environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Dissolve 21.7g of L-histidine (II) in 210ml of water, adjust the pH to 9.0 with trimethylamine; add 24.6g of tetrahydro-1,3-thiazine-2,6-bis in batches within 40 minutes at 10°C Ketone (III), and use trimethylamine to control the pH to 8.5, continue to stir and react for 30 minutes after the addition is complete; use formic acid to adjust the pH of the reaction solution to 4.2, stir until no bubbles are formed, and then use trimethylamine to adjust the pH to 8.2; dehydration under reduced pressure until transparent Viscous liquid, cooled to room temperature; add 1200ml of absolute ethanol, stir until white precipitate appears, filter with suction, successively soak with 95% ethanol, dichloromethane, ether, filter, dry to obtain 27.9g of white powdery solid, collected The yield is 88.1%, and the melting point is 243-247°C.
Embodiment 2
[0024] Dissolve 21.7g of II in 210ml of water, adjust the pH to 9.0 with trimethylamine; add 24.6g of III in batches within 40 minutes at 25°C, and control the pH to 8.5 with trimethylamine, and continue to stir for 30 minutes after the addition; adjust the pH with formic acid 4.2, Stir until no bubbles are formed, then adjust the pH to 8.2 with trimethylamine; dehydrate under reduced pressure until it becomes a transparent viscous liquid, cool to room temperature; add 1200ml of absolute ethanol, stir until white precipitate appears, filter with 95% Soak in ethanol, dichloromethane and ether, filter, and dry to obtain 23.6 g of white powdery solid, yield 74.5%, melting point: 240-245°C.
Embodiment 3
[0026] Dissolve 21.7g of II in 210ml of water, adjust the pH to 9.0 with trimethylamine; add 24.6g of III in batches within 40 minutes at 0°C, and control the pH to 8.5 with trimethylamine, and continue stirring for 30 minutes after the addition; adjust the pH with formic acid 4.2, Stir until no bubbles are formed, then adjust the pH to 8.2 with trimethylamine; dehydrate under reduced pressure until it becomes a transparent viscous liquid, cool to room temperature; add 1200ml of absolute ethanol, stir until white precipitate appears, filter with 95% Soak in ethanol, dichloromethane, and ether, filter, and dry to obtain 20.1 g of white powdery solid, yield 63.5%, melting point: 240-244°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com