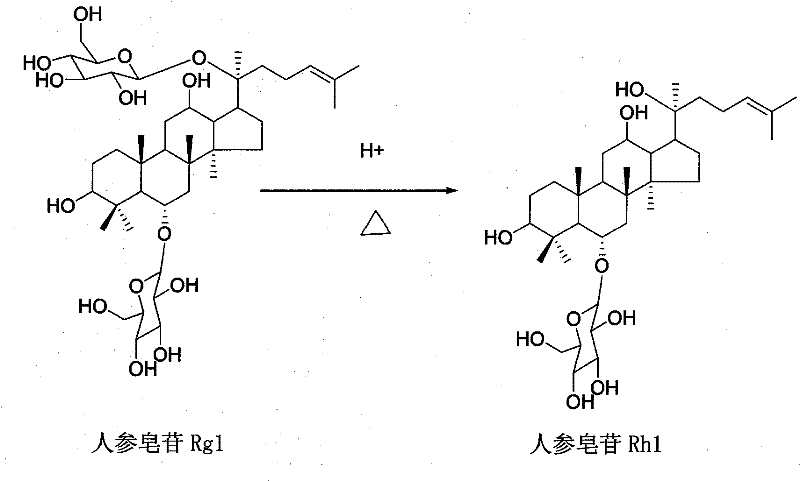
Method for preparing ginsenoside Rh1
A technology of ginsenoside and methanol, which is applied in the field of preparation of ginsenoside Rh1, can solve the problems of no further separation and purification of ginsenoside, no high-purity ginsenoside Rh1, and increased processing procedures, so as to save cost and energy, and facilitate industrial production , the effect of simplifying the separation procedure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] A. Acid hydrolysis: Dissolve the triol group ginsenosides with a purity greater than 80% in 8 times the amount of water, add glacial acetic acid to make the concentration of glacial acetic acid reach 15%, put the above mixed solution in a water bath, and hydrolyze at 50°C 7 hours.
[0018] B. Deacidification: the hydrolyzate is cooled to room temperature, and deacidified by DM130 macroporous adsorption resin column chromatography. Elute with water at a flow rate of 8 ml / min until the effluent is neutral, then elute with 85% ethanol until no purple spots are detected by thin-layer chromatography.
[0019] C. Enrichment: the hydrolyzate after deacidification is concentrated, dried, dissolved with 68% methanol, added 2 times the amount of silica gel to mix the sample, evaporated methanol, dry-packed, separated by silica gel column chromatography, and separated by chloroform:methanol : water=8:2:0.2 as the mobile phase, develop and elute. Thin-layer chromatography trackin...
Embodiment 2
[0022] A. Acid hydrolysis: Dissolve the triol group ginsenosides with a purity greater than 80% in 7 times the amount of water, add glacial acetic acid to make the concentration of glacial acetic acid reach 25%, put the above mixed solution in a water bath, and hydrolyze at 70°C 5 hours.
[0023] B, deacidification: the hydrolyzate is cooled to room temperature, deacidification with D101 macroporous adsorption resin column chromatography, water elution, flow rate 10ml / min, until the effluent is neutral, be 88% ethanol elution with concentration, until There was no purple spot detected by TLC.
[0024] C. Enrichment: the hydrolyzate after deacidification is concentrated, dried, dissolved with 70% methanol, added 2 times the amount of silica gel to mix the sample, evaporated methanol, dry-packed, separated by silica gel column chromatography, and separated by chloroform:methanol : water = 8: 2: 0.2 as the mobile phase, developing, elution, T thin layer chromatography tracking d...
Embodiment 3
[0027] A. Acid hydrolysis: Dissolve ginsenosides of the triol group with a purity greater than 80% in 9 times the amount of water, add glacial acetic acid to make the concentration of glacial acetic acid reach 18%, put the above mixed solution in a water bath, and hydrolyze at 58°C 6 hours.
[0028] B. Deacidification: Cool the hydrolyzate to room temperature, deacidify with DM130 macroporous resin column chromatography, elute with water at a flow rate of 11ml / min, until the effluent is neutral, rinse with 90% ethanol to a thin layer Chromatographic detection without purple spots.
[0029] C. Enrichment: the hydrolyzate after deacidification is concentrated, dried, dissolved with 71% methanol, added 2 times the amount of silica gel to mix the sample, evaporated methanol, dry-packed, separated by silica gel column chromatography, and separated by chloroform:methanol : Water = 8: 2: 0.2 as the mobile phase, developed, eluted, tracked and detected by thin-layer chromatography, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com