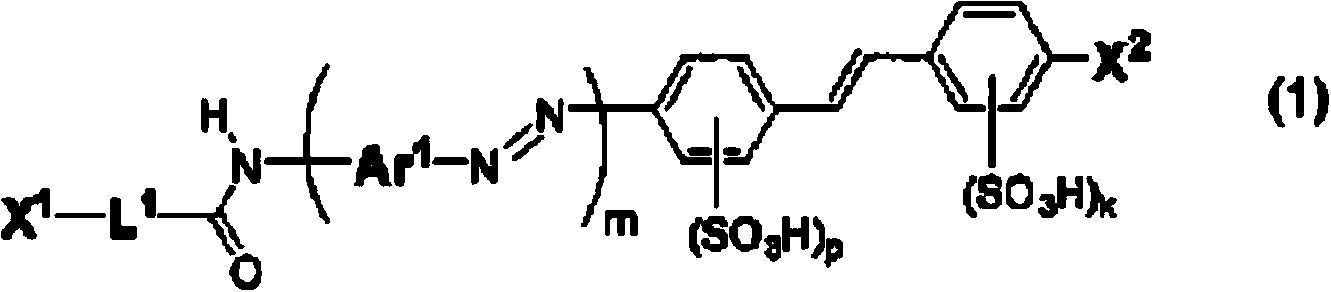
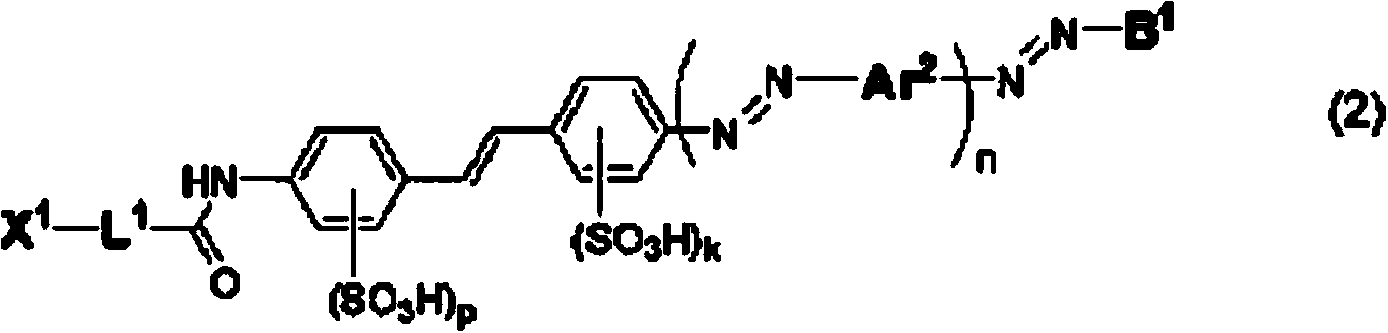
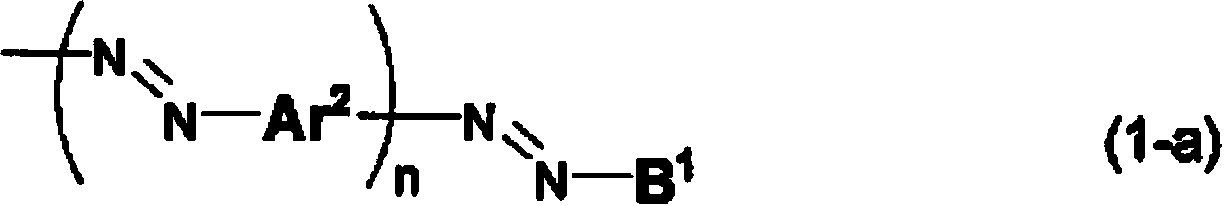Compound for anisotropic film
An anisotropic film and compound technology, which is applied in the field of anisotropic film compounds, can solve the problems of low dichroism, long molecular length and uneven screen edges of polarizing films, and achieves high dichroism and durability. excellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0383] Dissolve 8.9 parts by weight of 4-amino-4'-nitrostilbene-2,2'-disodium sulfonate in 90 parts by weight of N-methylpyrrolidone, add 1.6 parts by weight of sodium carbonate and 5.0 parts by weight of Parts of cinnamoyl chloride were reacted at 60°C for 30 minutes. After the reaction is terminated, it is discharged into water for salting out, and the precipitate generated by salting out is obtained by filtration. The precipitate was suspended in 115 parts by weight of water, 2.4 parts by weight of sodium hydrosulfide (70% commercially available) was added, and the mixture was reacted at 60° C. for 30 minutes. After the reaction was terminated, the sodium salt of the compound represented by the following formula (2-1a) was obtained by salting out.
[0384]
[0385] 5 parts by weight of the sodium salt of the compound represented by the above formula (2-1a) was dissolved in 100 parts by weight of water. Add 0.73 parts by weight of sodium nitrite under hydrochloric...
Embodiment 2
[0389] In the same manner as in Example 1, 4-amino-4'-nitrostilbene-2,2'-disulfonic acid disodium was acylated with cinnamic acid, and similarly reduced with sodium hydrosulfide to obtain A sodium salt of the compound represented by the above formula (2-1a). Next, a diazotization reaction was performed in the same manner, and 1.08 parts by weight of o-cresol was coupled under basic conditions, followed by salting out to obtain the sodium salt of the target compound represented by the following formula (2-2).
[0390] The maximum absorption wavelength (λmax) of this compound in a 10 ppm aqueous solution was 389 nm.
[0391]
Embodiment 3
[0393] 8.9 parts by weight of disodium 4-amino-4'-nitrostilbene-2,2'-disulfonate were dissolved in 90 parts by weight of water. Add 1.5 parts by weight of sodium nitrite under acidic conditions of hydrochloric acid for diazotization, and couple with 1.9 parts by weight of phenol under alkaline conditions, and through salting out, obtain the sodium salt of the compound represented by the following formula (2-3a) .
[0394]
[0395] 5 parts by weight of the sodium salt of the compound represented by the above formula (2-3a) was dissolved in 100 parts by weight of water. Next, 4 parts by weight of sodium hydroxide and 2 parts by weight of dimethyl sulfate were added and reacted at 90° C. for 6 hours. After the reaction is terminated, salting out is carried out, and the precipitate generated by the salting out is obtained by filtration. The precipitate was suspended in 100 parts by weight of water, 1.2 parts by weight of sodium hydrosulfide (70% commercially available)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| absorption wavelength | aaaaa | aaaaa |
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com