Carbonyl sulfide hydrolysis and preparation method thereof
A hydrolysis catalyst, catalyst technology, applied in catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., to achieve the effect of high desulfurization accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
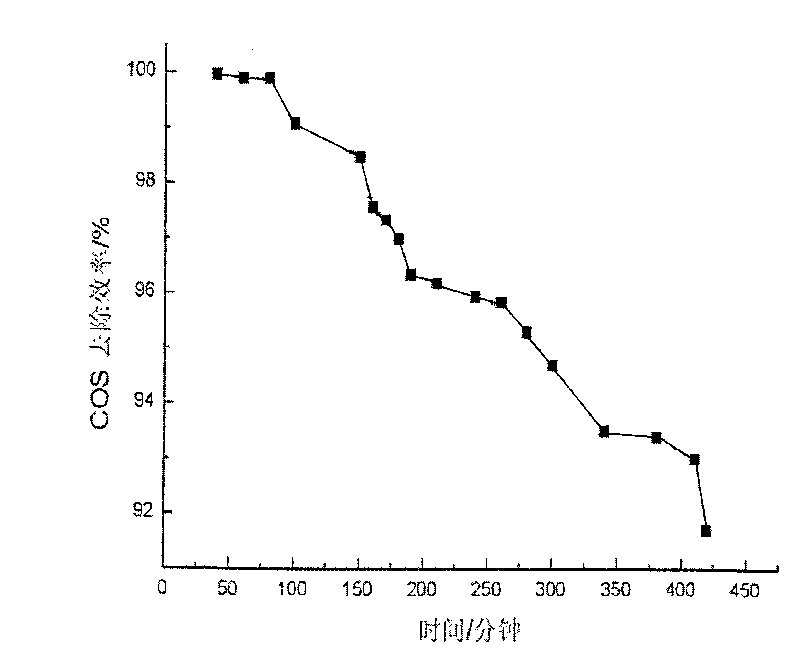
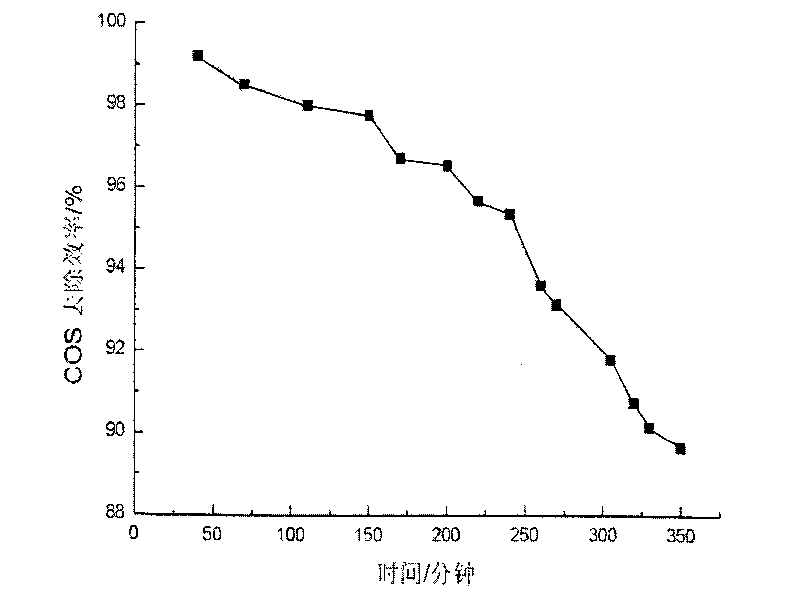
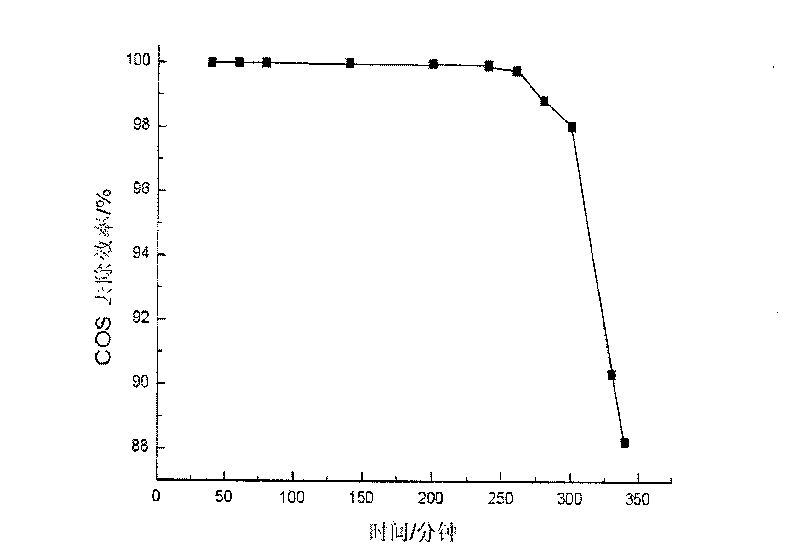
Image
Examples
Embodiment 1
[0026] (1) Weigh 8.1788g Ni(NO 3 ) 2 ·6H 2 O, 7.2115g Mg(NO 3 ) 2 ·6H 2 O and 7.575gFe(NO 3 ) 3 9H 2 O was dissolved in distilled water to make solution A, so that the total metal mole was 0.075mol; with NaOH, Na 2 CO 3 As a precipitating agent, take 5.2995g Na 2 CO 3 (0.05mol) and 6.75g NaOH were dissolved in distilled water to form solution B;
[0027] (2) Transfer the prepared A solution to the separatory funnel, drop the A solution into the B solution at a rate of 3.6mL / min under room temperature and mechanical stirring, control the pH of the solution at the end of the dropping to 9, drop After the addition, the stirring was continued for 30 minutes, and finally a suspension was obtained;
[0028] (3) crystallize the suspension obtained in step (2) in a water bath at 50° C. for 12 hours;
[0029] (4) suction filter the product obtained by crystallization, and wash it with distilled water to make it neutral; put the obtained product in an oven and dry at a temp...
Embodiment 2
[0032] (1) Weigh 4.3654g Co(NO 3 ) 2 ·6H 2 O, 8.724g Ni(NO 3 ) 2 ·6H 2 O and 11.2539gAl(NO 3 ) 3 9H 2 O was dissolved in distilled water to make solution A, so that the total metal mole was 0.075mol; with NaOH, Na 2 CO 3 As a precipitating agent, take 5.2995g Na 2 CO 3 (0.05mol) and 7.2g NaOH were dissolved in distilled water to form solution B;
[0033] (2) Transfer the prepared A solution to the separatory funnel, drop the A solution into the B solution at a rate of 3.6mL / min under room temperature and mechanical stirring, control the pH of the solution at the end of the dropping to 10, drop After the addition, the stirring was continued for 30 minutes, and finally a suspension was obtained;
[0034] (3) crystallize the suspension obtained in step (2) in a water bath at 60° C. for 12 hours;
[0035] (4) suction filter the product obtained by crystallization, and wash it with distilled water to make it neutral; put the obtained product in an oven and dry at a tem...
Embodiment 3
[0038] (1) Weigh 2.718g Cu(NO 3 ) 2 ·3H 2 O, 7.2115g Mg(NO 3 ) 2 ·6H 2 O and 7.575gAl(NO 3 ) 3 9H 2 O was dissolved in distilled water to make solution A, so that the total metal mole was 0.075mol; with NaOH, Na 2 CO 3 As a precipitating agent, take 5.2995g Na 2 CO 3 (0.05mol) and 6.75g NaOH were dissolved in distilled water to form solution B;
[0039] (2) Transfer the prepared A solution to the separatory funnel, drop the A solution into the B solution at a rate of 3.6mL / min under room temperature and mechanical stirring, control the pH of the solution at the end point of the addition to be 8, drop After the addition, the stirring was continued for 30 minutes, and finally a suspension was obtained;
[0040] (3) crystallize the suspension obtained in step (2) in a water bath at 40° C. for 12 hours;
[0041] (4) suction filter the product obtained by crystallization, and wash it with distilled water to make it neutral; put the obtained product in an oven and dry a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com