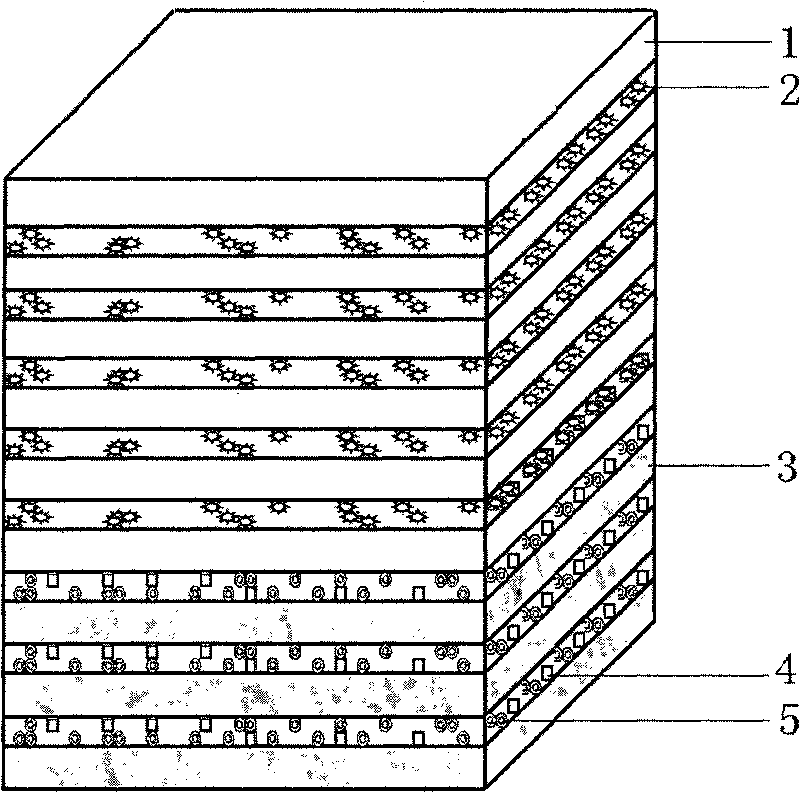
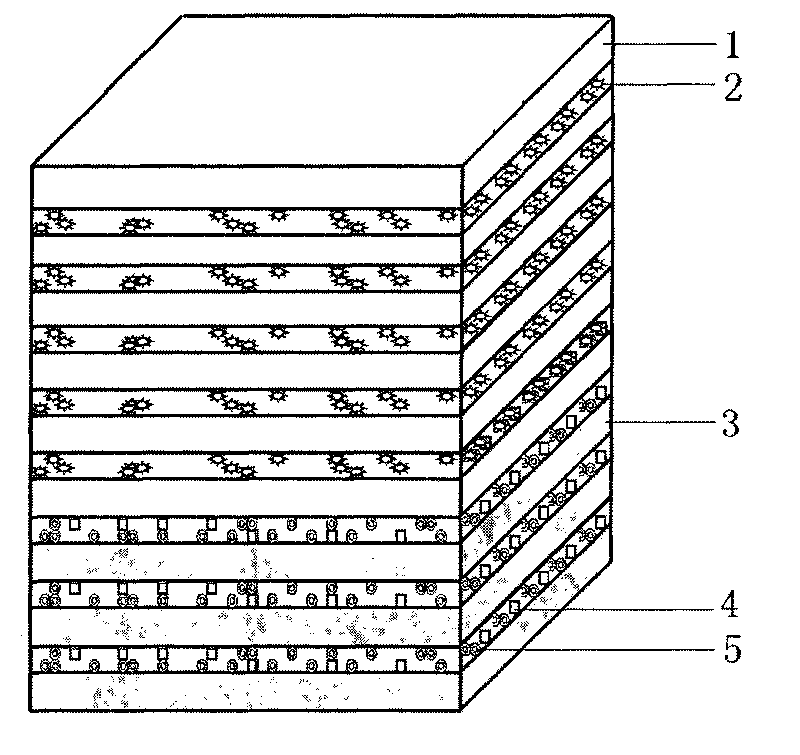
Artificial dura mater with bioactivity and preparation method thereof
An artificial dura mater, bioactive technology, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] (1) Preparation of electrospinning solution, drug-containing hydrosol solution and cross-linking agent solution:
[0076] For the electrospinning solution, hydrophobic L-polylactic acid and ε-caprolactone are selected, and the ratio of the two is 50:50. As a copolymerized polymer material, the number average molecular weight is 260,000, and it is soluble in hexafluoroisopropanol.
[0077] The cross-linking agent solution is 0.1M calcium chloride solution.
[0078] The hydrosol solution containing cytokines adopts the hemostatic factor alginate solution, and the mass percent concentration of the hemostatic factor in the cytokine alginate solution is 10 ppm.
[0079] Put the prepared 0.1M calcium chloride solution into a cell culture dish with a diameter of 150 mm, and place it on the flat receiver shared by the electrospinning device and the printer. Refit the Hewlett-Packard 550C inkjet printer according to the existing patent report, and fix it directly under the elec...
Embodiment 2
[0097] 1. Preparation of anti-adhesion layer
[0098] (1) Preparation of electrospinning solution, drug-containing hydrosol solution and cross-linking agent solution:
[0099] For the electrospinning solution, hydrophobic L-polylactic acid and ε-caprolactone are selected, and the ratio of the two is 50:50. As a copolymerized polymer material, the number average molecular weight is 260,000, and it is soluble in hexafluoroisopropanol.
[0100] The cross-linking agent solution is 0.1M calcium chloride solution.
[0101] The hydrosol solution containing cytokines adopts the hemostatic factor alginate solution, and the mass percent concentration of the hemostatic factor in the cytokine alginate solution is 10 ppm.
[0102] Put the prepared 0.1M calcium chloride solution into a cell culture dish with a diameter of 150 mm, and place it on the flat receiver shared by the electrospinning device and the printer. Refit the Hewlett-Packard 550C inkjet printer according to the existing p...
Embodiment 3
[0131] Carry out dog animal experiment with the dura mater that embodiment 1 makes:
[0132] There are 5 experimental dogs, weighing 15-20KG, aged 1.5-2 years, male or female. Intramuscular injection of ketamine was used for general anesthesia, and after anesthesia and hair removal, the animals were placed on a special operating table in the abdominal recumbent position. Disinfect with 2% iodine and 75% alcohol. The center of the head of the animal was cut longitudinally. The periosteum was separated with a stripper, the double top skull plate was exposed, and the skull was ground with a high-speed drill, and the double top was used to form a bone window. Use small scissors to cut off a rectangular dura mater with a size of 3 cm × 3 cm on the top of both sides to create a top dura mater defect. Electrocautery was performed on the exposed brain surface to cause six lesions of 1mm×1mm in size. The artificial meninges prepared in Example 1 of the present invention were trimme...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com