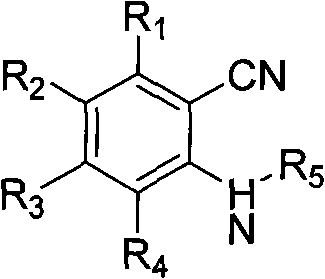
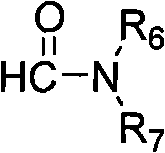
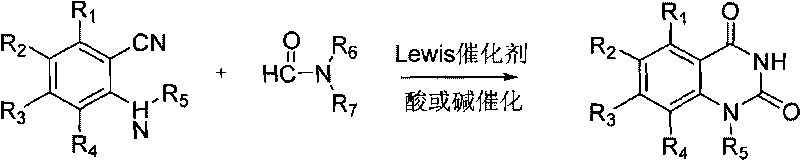
Method for synthesizing 1H,3H-quinazoline-2,4-diketone
A quinazoline and diketone technology, applied in the field of 1H synthesis, can solve the problems of expensive reagents, complicated operation, and high toxicity, and achieve the effects of increased yield, reduced pollution, and reduced damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 2-Aminobenzonitrile (0.3g, 0.002mol), DMF (8.0mL), anhydrous zinc chloride (0.27g, 0.002mol), the mixture was added to the autoclave, 0.1ml of concentrated hydrochloric acid was added dropwise, oil Stir in the bath and heat to 190°C for 5 hours. Heating was stopped, the reaction was completed, and left to cool to room temperature. The reaction solution was diluted with water, and a white precipitate precipitated out. Suction filtration, water washing. Then dissolve it with 1,4-dioxane, and then filter while it is hot to remove insoluble impurities. The obtained filtrate was subjected to rotary evaporation under reduced pressure to obtain a light yellow solid, which was then recrystallized from a mixed solvent of methanol and tetrahydrofuran, with a yield of 95%, m.p.>300°C. The structural characterization data of the reaction formula and its products are as follows:
[0026]
[0027] Structural characterization data of the product:
[0028] IR(KBr), σ / cm -1 : 3...
Embodiment 2
[0033] 2-Aminobenzonitrile (0.59g, 0.005mol), DMF (8.0mL), anhydrous zinc chloride (0.67g, 0.005mol), the mixture was added to the autoclave, and then 0.04gCH 3 ONa, stirred in an oil bath and heated to 190°C for 5 hours. Heating was stopped, the reaction was completed, and left to cool to room temperature. The reaction solution was diluted with water, and a white precipitate precipitated out. Suction filtration, water washing. Then dissolve it with 1,4-dioxane, and then filter while it is hot to remove insoluble impurities. The obtained filtrate was rotary evaporated under reduced pressure to obtain a light yellow solid, which was then recrystallized from a mixed solvent of methanol and tetrahydrofuran, with a yield of 93%, m.p.>300°C.
Embodiment 3
[0035] 2-Aminobenzonitrile (0.59g, 0.005mol), DMF (8.0mL), anhydrous copper chloride (0.67g, 0.005mol), the mixture was added to the autoclave, 0.1ml of concentrated hydrochloric acid was added dropwise, oil Stir in the bath and heat to 190°C for 5 hours. Heating was stopped, the reaction was completed, and left to cool to room temperature. The reaction solution was diluted with water, and a white precipitate precipitated out. Suction filtration, water washing. Then dissolve it with 1,4-dioxane, and then filter while it is hot to remove insoluble impurities. The obtained filtrate was rotary evaporated under reduced pressure to obtain a light yellow solid, which was then recrystallized from a mixed solvent of methanol and tetrahydrofuran, with a yield of 78%, m.p.>300°C.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap