Medicament for inhibiting asodilatatioin
A technology of vasodilation and medicine, which is applied in the field of medicines for preparing its main active ingredients from P. loropetalum, and can solve problems such as unseen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 The extraction of the crude extract of Pyracha chinensis
[0030] The crude matter of P. saponifera was extracted by biological enzymatic hydrolysis. The specific process is: raw material sampling analysis and testing before the production line → raw material acceptance → deep cleaning → enzymatic hydrolysis of combined bacteria → inactivation → dynamic temperature immersion extraction → low-temperature concentration → instant freezing → high-speed centrifugal sedimentation separation → spray drying → packaging inspection → Baihuahua Crude wood extract.
[0031] The specific extraction method is to crush the fresh material of P. chinensis. The biobroken product of P. candidum was obtained by enzymatic hydrolysis with combined bacteria, extracted by dynamic warm soaking method (temperature 85°C, time 120m, pH value 6.5±0.2), warm soaking extraction time was 16 hours, and the extract was obtained. First use alcohol extraction and water precipitation method to ...
Embodiment 2
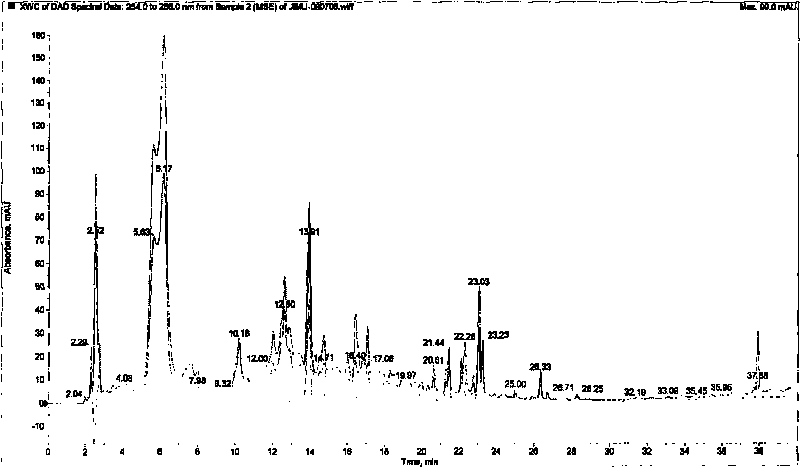
[0035] Example 2 Preliminary Analysis Report on Chemical Components of P.
[0036] 1. Sample preparation
[0037] Finely weigh 1.06 mg of crude powder, dissolve in 1 mL of 50% methanol, and pass through a 0.45 μm microporous membrane to obtain the product.
[0038] 2. Chromatographic conditions
[0039] High-performance liquid chromatography: Agilent 1200 series high-performance liquid chromatography (Agilent Technologies, USA), including a diode array detector, a quaternary gradient pump, an online degasser, and an autosampler.
[0040] Column: YMC-Pack Pro C 18 (150mm×3.0mm ID, 5μm);
[0041] Mobile phase: A (methanol), B (0.1% formic acid H 2 O)
[0042] Gradient elution (0→35min, A: 10%→98%; 35min-40min, A: 98%→98%;);
[0043] Flow rate 0.3mL / min;
[0044] UV scanning range: 200-400nm;
[0045] Detection wavelength: 200-400nm; Column temperature: 40°C.
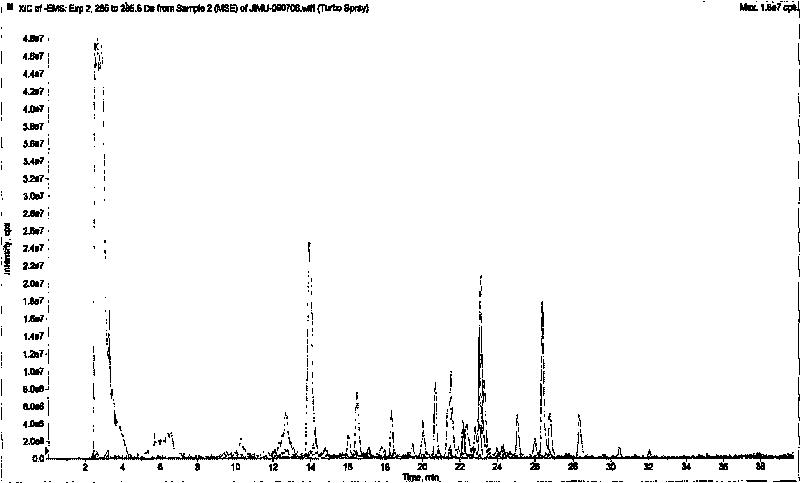
[0046] 3. Mass Spectrometry Conditions
[0047] 1. Mass spectrometer: QTRAP TM Type quadrupole-linear ion tra...
Embodiment 3
[0069] Example 3 Effects of the crude extract of P. chinensis on the vasodilation induced by bradykinin in rats
[0070] 1. Materials
[0071] Animals: Wistar rats, male, weighing 250 g, provided by the Institute of Experimental Animals, Chinese Academy of Medical Sciences, certificate number, SCXK (Beijing) 2008-0004.
[0072] Tested product: Crude extract of P. chinensis, brown powdery solid, provided by Jiangxi Deyu Group. Prepare to the required concentration with normal saline before use.
[0073] Experimental liquid:
[0074] 1) KH solution (in 2000ml distilled water, add NaCl: 13.7918g; KCl: 0.7008g; CaCl2: 0.56605g; anhydrous MgSO4: 0.2841g; KH2PO4: 0.3212g; NaHCO3: 4.1803g; Glucose: 4.3994g. magnetic stirring, spare)
[0075] 2) Bradykinin with a final concentration of 3 μmol / L, prepared with physiological saline, stored at -20°C;
[0076] 3) Potassium chloride solution with a final concentration of 60mmol / L.
[0077] 2. Method
[0078] 1) Rats were anesthetize...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com