Method for preparing barium ferrite with low coercive force temperature coefficient
A technology of barium ferrite and temperature coefficient, applied in the field of preparation of barium ferrite with low temperature coefficient of coercivity, can solve the problems of large temperature coefficient of coercivity, large temperature change of coercivity, etc. Chance of reunion, good crystal phase, energy saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
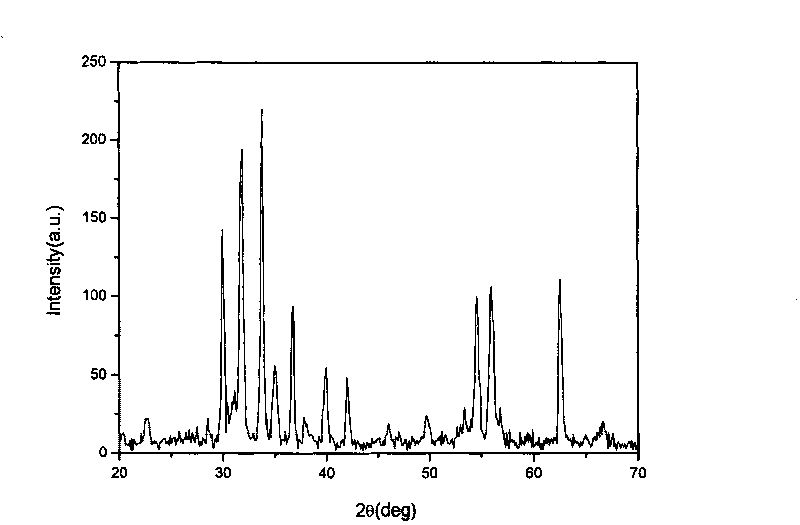
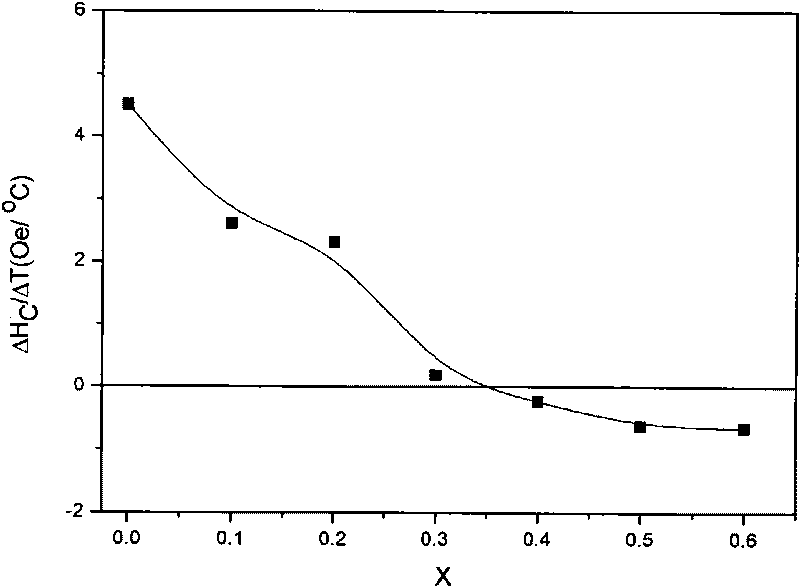
[0017] see Figure 1-Figure 2 , by BaFe 12-2x co x / 2 Zn x / 2 sn x o 19 Molecular formula weighs 1×10 -2 mol BaCl 2 2H 2 O, 9.17×10 -2 mol FeCl 3 ·6H 2 O, 4.58×10 -3 mol ZnCl 2 , 4.58×10 -3 mol CoCl 2 ·6H 2 O, 9.17×10 -3 mol SnCl 2 2H 2 O was dissolved in distilled water with stirring in a 70°C water bath to form a mixed solution. Take another 2.83×10 -1 mol of anhydrous sodium carbonate was dissolved in an appropriate amount of distilled water and stirred in a water bath at 70°C. Quickly pour the above mixed solution into the continuously stirring sodium carbonate solution, continue to stir for 1 hour, cool and age, filter with suction, and wash with water until there is no Cl - , dried at 80°C. Weigh 2 g of the precipitate, grind and mix with potassium chloride solid in a ratio of 1:1 by weight, heat up to 450°C for 2 hours at a heating rate of 15°C / min, and heat up to 950°C for 4 hours; The heat-treated product is then washed with water to be free of Cl ...
Embodiment 2
[0019] see Figure 4-Figure 5 , by BaFe 12-2x co x / 2 mn x / 2 sn x o 19 Molecular formula weighs 1×10 -2 mol BaCl 2 2H 2 O, 1.06×10 -1 mol FeCl 3 ·6H 2 O, 9.14×10 -4 mol ZnCl 2 , 9.14×10 -4 mol CoCl 2 ·6H 2 O, 1.83×10 -3 mol SnCl 2 2H 2 O was dissolved in distilled water with stirring in a 70°C water bath to form a mixed solution. Take another 2.83×10 -1 mol of anhydrous sodium carbonate was dissolved in an appropriate amount of distilled water and stirred in a water bath at 70°C. Quickly pour the above mixed solution into the continuously stirring sodium carbonate solution, continue to stir for 1 hour, cool and age, filter with suction, and wash with water until there is no Cl - , dried at 80°C. Weigh 2g of the precipitate, grind and mix it with potassium chloride solid at a weight ratio of 1:1, and conduct heat treatment at a heating rate of 15°C / min at (450°C, 2h)+(950°C, 4h). The heat-treated product is then washed with water to be free of Cl - , dried...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com