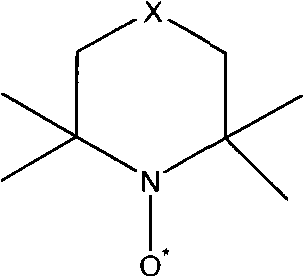
Method for synthesizing 3-methyl-2-butene aldehyde
A synthetic method and technology of crotonaldehyde, which is applied in the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of many by-products and poor selectivity, and achieve less side reactions, less environmental pollution, and The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] In a 250ml three-necked round bottom flask equipped with magnetic stirring, 12.9g (0.15mol) of 3-methyl-2-butenol and 100ml of dichloromethane as a solvent were added, and replaced by nitrogen gas. Add 2.0 g (5 mmol) of ferric nitrate nonahydrate and 0.71 g (4.5 mmol) of TEMPO, and slowly introduce pure oxygen at 25°C. After reacting for about 6 hours, the conversion rate of 3-methyl-2-butenol was 99%. The obtained reaction liquid was filtered, and the solvent was distilled off to obtain 11.9 g of 3-methyl-2-butenal (the content detected by gas chromatography was 97.7%), and the yield was 92.3%.
Embodiment 2
[0022] In a 250ml three-neck round bottom flask equipped with magnetic stirring, 12.9g (0.15mol) of 3-methyl-2-butenol and 100ml of solvent toluene were added, and replaced by nitrogen gas. Add 1.4 g (5 mmol) of cobalt nitrate and 0.71 g (4.5 mmol) of 4-OCH3-TEMPO, and slowly introduce air at 70°C. After reacting for about 14 hours, the conversion rate of 3-methyl-2-butenol was 95%. The reaction solution was filtered, and the solvent was distilled off to obtain 10.8 g of 3-methyl-2-butenal (content: 95.2%), with a yield of 81.6%.
Embodiment 3
[0024] In a 250ml three-necked round bottom flask equipped with magnetic stirring, 12.9g (0.15mol) of 3-methyl-2-butenol and 100ml of solvent cyclohexane were added, and replaced by nitrogen gas. Add 0.84 g (3 mmol) of manganese nitrate and 0.51 g (3 mmol) of 4-O-TEMPO, and slowly introduce air at 20°C. After reacting for about 8 hours, the conversion rate of 3-methyl-2-butenol was 97%. The resulting reaction solution was filtered, and the solvent was evaporated to obtain 11.3 g of 3-methyl-2-butenal (content 96.8%), with a yield of 86.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com