Dispersing tablet containing tegafur, gimeracil and oteracil potassium
A technology of oteracil potassium and gimeracil, which is applied in the field of western medicine preparations, can solve the problems of increasing the complexity of the process, the inability to ensure the mixing uniformity of tegafur drug-loaded microspheres, and uneven tablet content.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1 The preparation of the dispersible tablet containing tegafur, gimeracil and oteracil potassium of the present invention
[0053] 1. Prescription
[0054] Name of raw material Amount / g
[0055]
[0057] Tegafur 20
[0058] Blank lactose core 40
[0059] Ethanol 200ml
[0060] water 300ml
[0061] Isolation layer:
[0062] Hypromellose 20
[0063] Triacetin 2.5
[0064] Talc 5
[0065] Coating layer:
[0066] Gimeracil 5.8
[0067] Oteracil Potassium 19.6
[0068] Hypromellose 20
[0069] Triacetin 2.5
[0070] Talc 5
[0071] Other accessories:
[0072] Crospovidone 20
[0073] Low-substituted hydroxypropyl methylcellulose 18
[0074] Orange Flavor 2
[0076] Sucrose 15
[0077] Lactose 79.6
[0078]
[0079] A total of 1000 pieces were made
[0080] 2. Preparation process
[0081] (1) The blank lacto...
Embodiment 2
[0088] Embodiment 2 Preparation of dispersible tablets containing tegafur, gimeracil and oteracil potassium of the present invention
[0089] 1. Prescription
[0090] Name of raw material Amount / g
[0091]
[0093] Tegafur 20
[0094] Blank lactose core 60
[0095] Ethanol 200ml
[0096] water 300ml
[0097] Isolation layer
[0098] Polyvinyl alcohol 20
[0099] Triacetin 2.5
[0100] Talc 5
[0101] Coating layer:
[0102] Gimeracil 5.8
[0103] Oteracil Potassium 19.6
[0104] Polyvinyl alcohol 20
[0105] Triacetin 2.5
[0106] Talc 5
[0107] Other accessories:
[0108] Croscarmellose Sodium 18
[0109] Low-substituted hydroxypropyl methylcellulose 20
[0110] Orange Flavor 2
[0112] Sucrose 15
[0113] Microcrystalline cellulose 59.6
[0114]
[0115] A total of 1000 pieces were made
[0116] 2. The ...
Embodiment 3-6
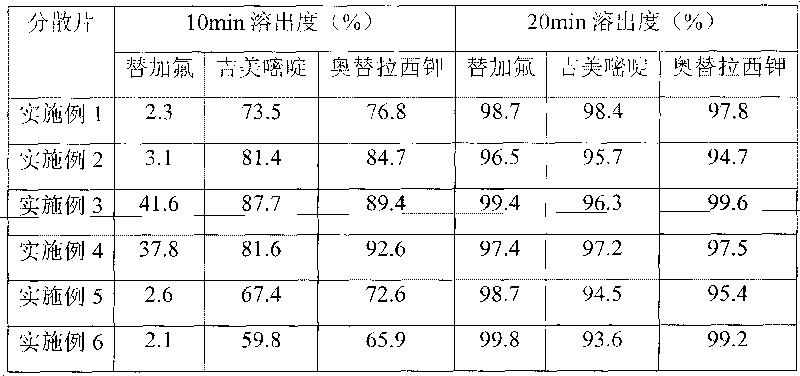
[0118] Prepare the drug-loaded pellets of tegafur according to the first step in Example 1, then prepare the coating solution according to the ingredients listed in Table 4, coat the tegafur pellets according to the process of Example 1, and press into tablets , the hardness of the dispersible tablet of Example 3-6 is between 45-65N. The weight gain of the isolation layer is 2%, 3%, 4%, and 10% in turn.
[0119] The preparation of table 4 Siggio dispersible tablets
[0120] Ingredients (g)
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com