Gadolinium-containing macromolecule magnetic resonance intravascular contrast medium and preparation method thereof
An angiography and magnetic resonance technology, applied in the field of magnetic resonance angiography contrast agent and its preparation, can solve the problems of incomplete clearance and accumulation toxicity, and achieve the effect of solving gadolinium accumulation and simple treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: The small-molecule gadolinium complex is gadolinium diethylenetriaminepentaacetate (Gd-DTPA), and the double metal hydroxide is Mg 2 Al(OH) 6 NO3 .
[0031] a. Add 15.42g Mg(NO 3 ) 2 ·6H 2 O and 7.48g Al(NO 3 ) 3 9H 2 O was dissolved in 50mL freshly boiled high-purity water to form solution A.
[0032] b. Add 5.25g NaNO 3 and 9.2mL NH 3 ·H 2 O was dissolved in 50mL freshly boiled high-purity water to form solution B;
[0033] c. Take 1.5 mL of solution A and add it to the organic phase containing nonylphenol polyoxyethylene ether, wherein the volume ratio of each substance in the organic phase is fixed as cyclohexane: nonylphenol polyoxyethylene ether: n-hexanol 20:3:3.
[0034] d. Take 1.5mL of solution B and 1.5mL of 2.0M ammonia water and add an equal volume of the above organic phase;
[0035] e. Add the microemulsion containing solution B dropwise into the microemulsion containing solution A, and heat to 80° C. for 8 hours after the additi...
Embodiment 2
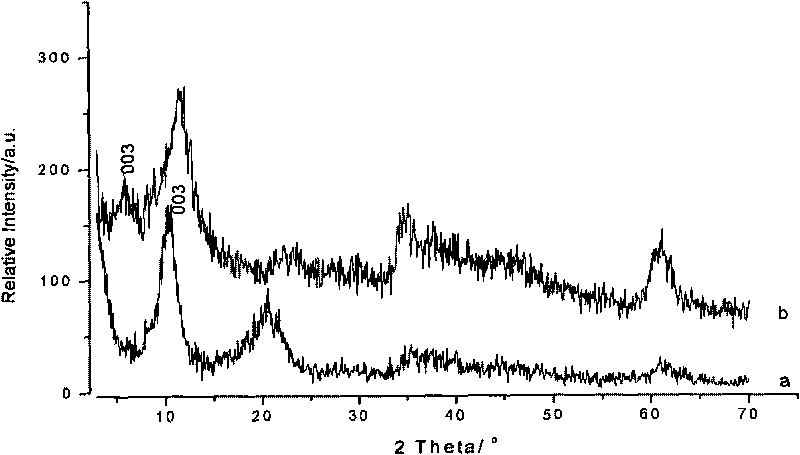
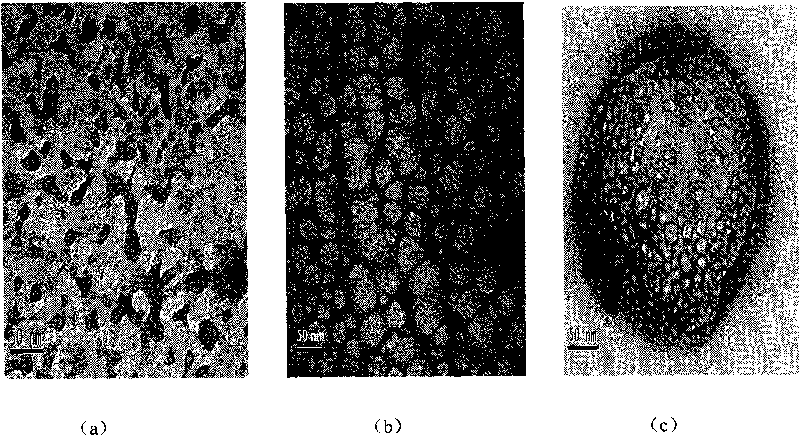
[0039] Embodiment two: the present embodiment is basically the same as embodiment one, and the difference is that the total volume of the aqueous solution added in each step of steps c, d, and f is increased to 2 times that of embodiment one, that is, after adding Na 2 [Gd(dtpa)H 2 O] After the solution was added, the volume ratio of the substances in the reaction system, cyclohexane: nonylphenol polyoxyethylene ether: n-hexanol: aqueous solution increased from 20:3:3:3 to 20:3:3:6. figure 2 It can be seen from the TEM photo in (b) that the particle size of the product composite of Example 2 has increased to 48 nanometers, and the particle size is relatively large. Longitudinal relaxation efficiency 4.0mM -1 the s -1 , transverse relaxation efficiency 5.5mM -1 the s -1 .
Embodiment 3
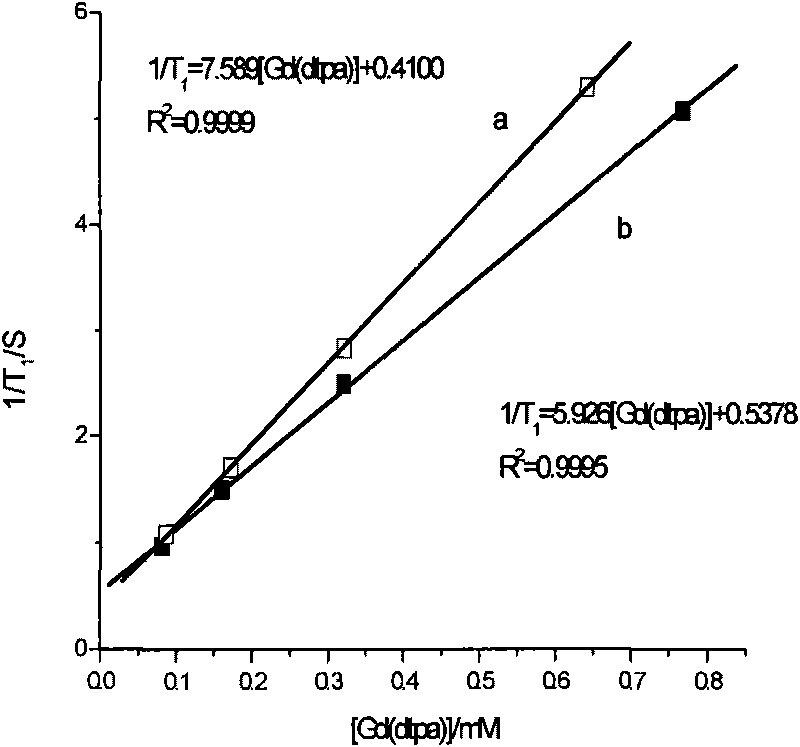
[0040] Embodiment three: this embodiment is basically the same as embodiment one, the difference is that step f is adjusted to add Na with a molar ratio of gadolinium to aluminum of 1:1 2 [Gd(dtpa)H 2 O] solution, that is, the amount of gadolinium added is 2 times that of Example 1, and the loading amount of Gadolinium in the product complex is increased to Gd%=5.94% from Gd%=5.41% of Example 1, but the longitudinal relaxation efficiency is relatively Embodiment 1 decreased to 5.9mM -1 the s -1 , see image 3 curve b; Figure 4 Curve b is its transverse relaxation efficiency.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com