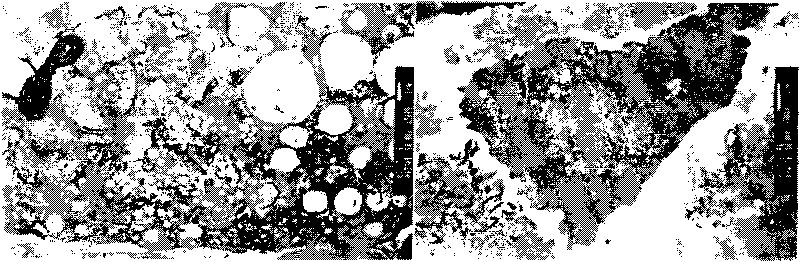
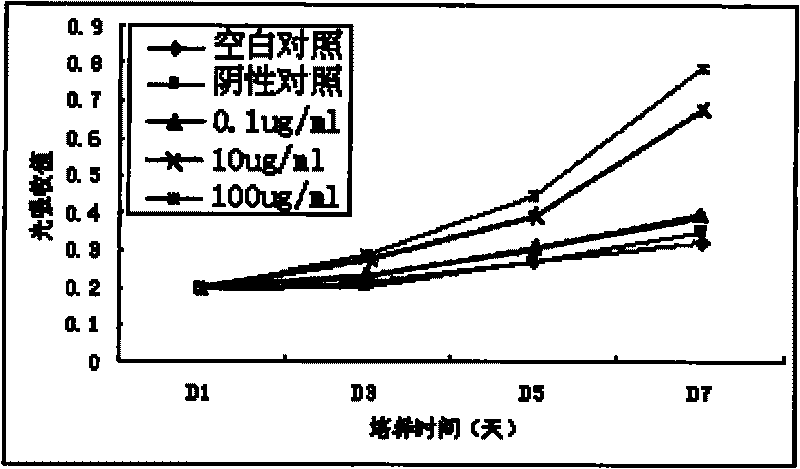
Application of human Keratiocyte growth factor 1 in preparation of medicament for treating foot ulcers and leg ulcers of diabetes
A technology of keratinocytes and growth factors, used in the treatment of diabetic foot and leg ulcers, the application field of drugs, can solve problems such as stimulating the growth of epithelial cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The preparation of embodiment 1 freeze-dried preparation of the present invention
[0019] Active ingredient: Recombinant human keratinocyte growth factor 1 (you can buy palifermin or kepivance from Amgen, USA) or use conventional gene recombination technology to produce recombinant human keratinocyte growth factor 1, the method is as follows:
[0020] a. According to methods well known to those skilled in the field of biotechnology, artificially synthesize or use RT-PCR technology to clone the cDNA sequence of recombinant human keratinocyte growth factor 1 [fibroblast growth factor 7 (keratinocyte growth factor), Homo sapiens , Gene ID 2252], construct a recombinant plasmid, and transfect the engineered bacteria E.coli to express, obtain the genetically engineered bacteria expressing recombinant human keratinocyte growth factor 1, and carry out the expression of recombinant human keratinocyte growth factor 1 by fermenting and cultivating the genetically engineered bact...
Embodiment 2
[0024] The preparation of embodiment 2 spray formulations of the present invention
[0025] In order to facilitate transportation and storage, recombinant human keratinocyte growth factor 1 is prepared into a freeze-dried preparation, which can be prepared as a spray preparation according to the need: dissolve the freeze-dried preparation with physiological saline or a common medical solvent, and the concentration range of the active substance after dissolution is 50ng~100ug / ml, put it into a sprayer that meets the requirements, and then spray it.
Embodiment 3
[0026] The preparation of embodiment 3 gel formulations of the present invention
[0027] In order to facilitate transportation and storage, recombinant human keratinocyte growth factor 1 is prepared as a freeze-dried preparation, which can be prepared as a gel preparation according to the needs when used. The preparation method is as follows:
[0028] With the characteristics of polyoxyethylene-polyoxypropylene block copolymer (Pluronic) being liquid at low temperature and condensed into gel at body temperature, it is formulated as a gel companion of recombinant human keratinocyte growth factor 1 freeze-dried powder, which is compatible with recombinant human The keratinocyte growth factor 1 freeze-dried powder is stored together at low temperature (4-8°C). When used, dissolve the freeze-dried powder with a gel companion, suck it out and apply it to the affected area. The gel will be formed to cover the affected area under the action of body temperature, and the recombinant hu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com