Temperature-sensitive microgel containing chiral side group and preparation method thereof
A temperature-sensitive, micro-gel technology, applied in the field of polymer materials, to achieve the effect of large adsorption capacity, strong reproducibility, and controllable reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
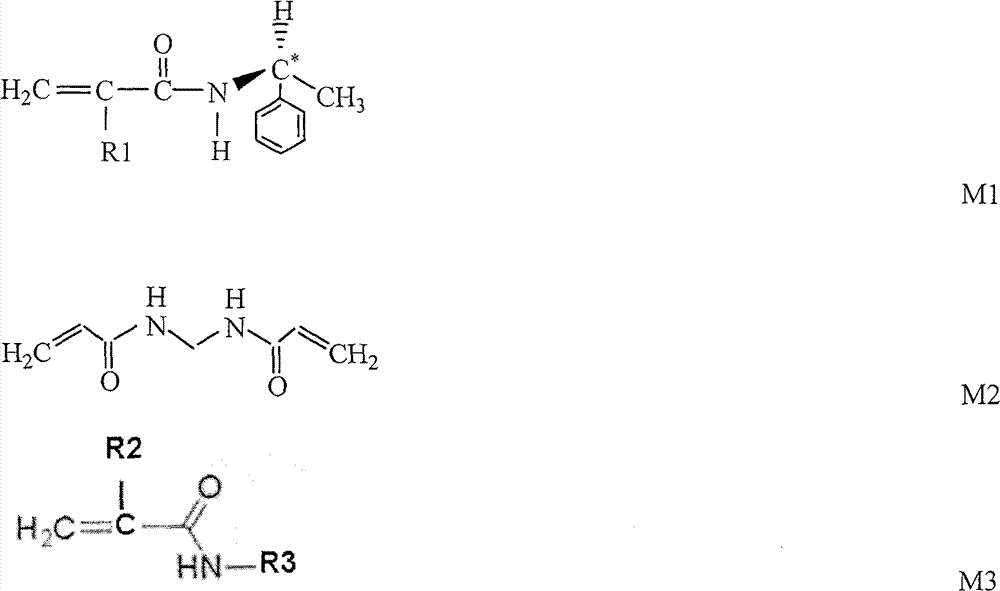
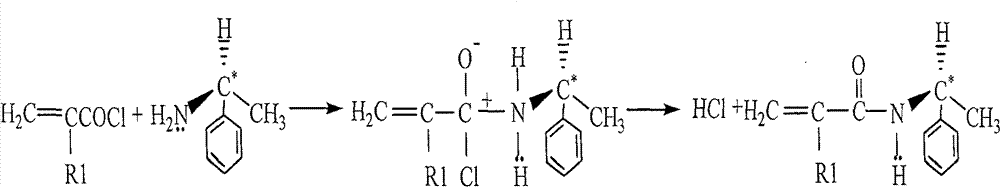
[0039] At 20°C, add 1.80g of S-(-)-α-phenylethylamine and 1.20g of triethylamine into 80mL of tetrahydrofuran in turn, stir magnetically for 20min to form a transparent solution, lower the temperature to -4°C within 10min, and add 1.4 mL of methacryloyl chloride, stirred for 3 hours, then naturally heated to 20°C and continued to stir, and after 2 hours, cooled down to -4°C again, added 230mL of ethyl acetate, washed and extracted with ice-cold 1mol / L hydrochloric acid, water, and saturated saline in sequence, Drying over anhydrous sodium sulfate, and obtaining S-(-)-α-phenethyl-methacrylamide after vacuum distillation;
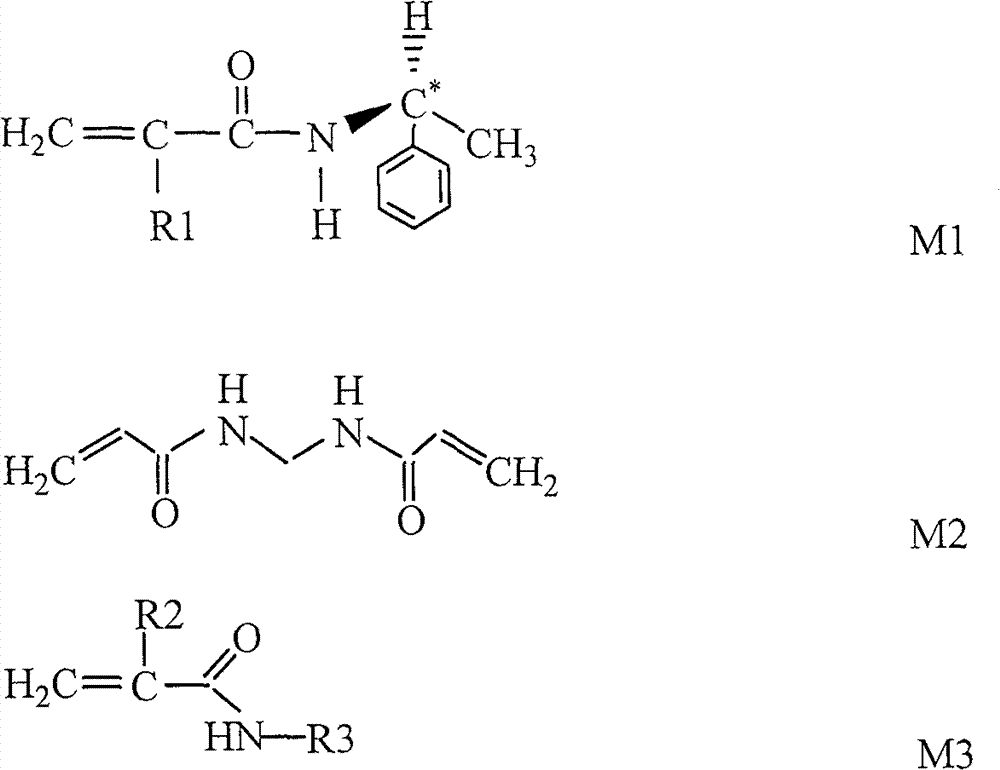
[0040] At room temperature, under nitrogen protection and stirring, 0.06g of S-(-)-α-phenylethyl-methacrylamide, 0.1g of methylenebisacrylamide, and 1.34g of isopropylacrylamide were sequentially added to tetrahydrofuran, Stir at 20°C for 50 minutes until completely dispersed to form a homogeneous solution;
[0041] Put the above mixed solution into a four-n...
Embodiment 2
[0044] At 25°C, add 1.65g of S-(-)-α-phenylethylamine and 1.45g of triethylamine into 60mL of tetrahydrofuran in turn, stir magnetically for 30min to form a transparent solution, lower the temperature to -5°C within 10min, and add 1.2 mL of acryloyl chloride, stirred for 2 hours, then naturally heated to 25°C and continued to stir, and after 2 hours, cooled down to -5°C again, added 180mL of ethyl acetate, washed and extracted with ice-cold 1mol / L hydrochloric acid, water, and saturated saline in sequence, and anhydrous Drying over sodium sulfate and distillation under reduced pressure to obtain S-(-)-α-phenethyl-acrylamide;
[0045] At room temperature, under nitrogen protection and stirring, add 0.10g of S-(-)-α-phenylethyl-acrylamide, 0.12g of methylenebisacrylamide, and 1.28g of isopropylacrylamide into tetrahydrofuran in sequence, at 25°C Stir for 40 minutes until completely dispersed to form a homogeneous solution;
[0046] Put the above mixed solution into a four-necke...
Embodiment 3
[0049] At 22°C, add 3.30g of S-(-)-α-phenylethylamine and 2.80g of triethylamine into 120mL of tetrahydrofuran in turn, stir magnetically for 30min to form a transparent solution, lower the temperature to -3°C within 10min, and add 2.0 mL of undecylenoyl chloride, stirred for 2 hours, then naturally heated to 22°C and continued to stir, after 2 hours, cooled down to -3°C again, added 200mL of ethyl acetate, washed and extracted with ice-cold 1mol / L hydrochloric acid, water, and saturated saline in sequence, Drying over anhydrous sodium sulfate, and obtaining S-(-)-α-phenethyl-undecylenamide after vacuum distillation;
[0050] At room temperature, under nitrogen protection and stirring, 0.02g of S-(-)-α-phenylethyl-undecylenamide, 0.10g of methylenebisacrylamide, and 1.38g of isopropylacrylamide were added to chloroform in sequence, Stir at 25°C for 40 minutes until completely dispersed to form a homogeneous solution;
[0051] Put the above mixed solution into a four-neck flas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com