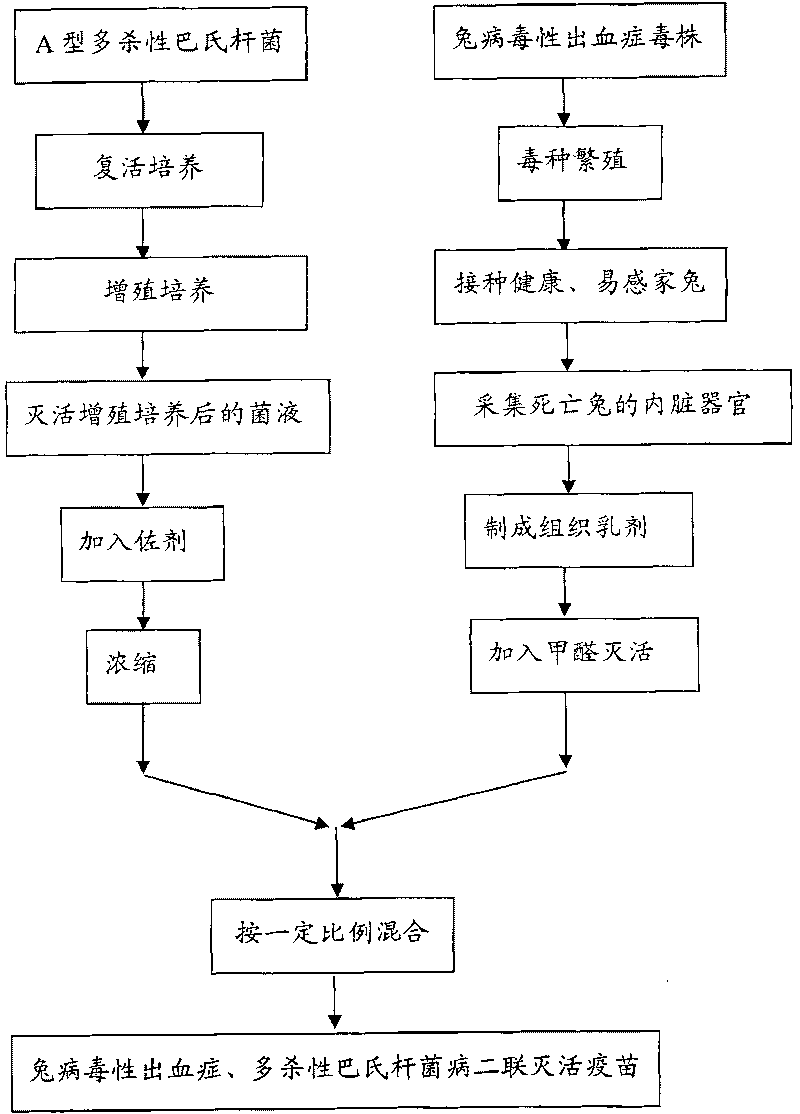
Method for preparing inactivated bivalent vaccine against rabbit hemorrhagic disease and pasteurella multocida disease
A technology of rabbit viral hemorrhage and double inactivated vaccine, which can be used in antiviral agents, viral antigen components, and medical preparations containing active ingredients, etc., and can solve problems such as threats to the healthy development of the rabbit industry and economic losses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Rabbit virus haemorrhagic disease CD 85-2 Strain subculture
[0044] The preserved rabbit viral hemorrhagic disease strain CD 85-2 Make 10-fold dilution with normal saline, and inoculate healthy antibody-negative rabbits weighing 1.5kg at the age of two months from unimmunized non-epidemic areas. Each subcutaneous injection of 1ml, the virus titer in the rabbit can be maintained at 2 10 Bacteria / ml or more. Aseptically collect rabbits with a weight of 1.5kg that died within 24-96 hours after inoculation, aseptically collect the heart, liver, lung, spleen or kidney with histopathological changes from rabbits that died within 1 hour, and remove them The connective tissue on the body, in this example, the liver is selected as the poisonous species.
[0045] CD 85-2 For inoculation, the heart, liver, lung, spleen or kidney of the dead rabbit whose connective tissue has been removed, preferably the liver is mashed with saline, and filtered to prepare a tissue emulsion w...
Embodiment 2
[0050] The preserved rabbit viral hemorrhagic disease strain CD 85-2 Make 10-fold dilution with normal saline, and inoculate 5-year-old healthy antibody-negative rabbits with a body weight of 2.0 kg from non-infested areas. Each subcutaneous injection of 1ml, the virus titer in the rabbit can be maintained at 2 10 above. Aseptically collect rabbits with a weight of 2.0kg that died within 24-96 hours after inoculation, aseptically collect the heart, liver, lung, spleen or kidney with histopathological changes from rabbits that died within 1 hour, and remove them In this case, the spleen is selected as the poisonous connective tissue.
[0051] CD 85-2 For inoculation, the heart, liver, lung, spleen or kidney of the dead rabbit whose connective tissue has been removed is selected and the spleen is mashed with normal saline, and filtered to prepare a tissue emulsion with a volume ratio of 1:9 between the mashed internal organs and normal saline. Then add formaldehyde solutions...
Embodiment 3
[0056] Vaccine Safety Test
[0057] Inoculate 1-month-old healthy susceptible rabbits with subcutaneous or intramuscular single-dose 1ml of the vaccine obtained according to the method of Example 1 and carry out safety test: each 4 healthy susceptible rabbits of 1-month-old were inoculated subcutaneously or intramuscularly with the trial-made vaccine, 1ml / only, in addition, inject 1ml / non-immune control of sterilized physiological saline to 4 rabbits at the same time, measure body temperature, observe for 10 days. Results After immunization, there was no abnormal mental state, normal appetite, no significant change in body temperature, and no adverse reactions such as inflammation at the injection site.
[0058] Safety test of subcutaneous or intramuscular single dose 1ml repeated inoculation of 1-month-old healthy susceptible rabbits: subcutaneously or intramuscularly inoculate 4 1-month-old healthy susceptible rabbits with 1ml each, and repeat the inoculation 7 days later I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com