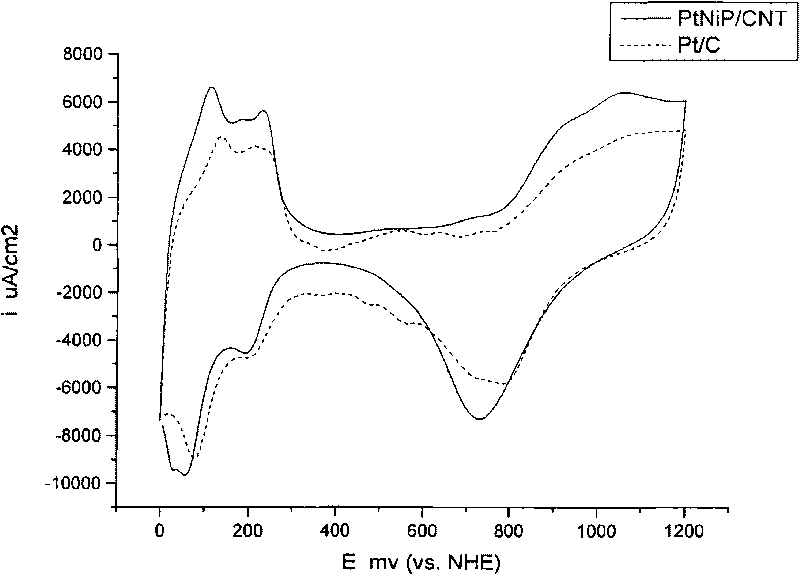
Preparation method of fuel-cell catalyst
A fuel cell and catalyst technology, applied in chemical instruments and methods, physical/chemical process catalysts, battery electrodes, etc., can solve the problems of reduction, catalyst performance reduction, unfavorable cost, etc., to prevent agglomeration, slow down the corrosion rate, prolong the The effect of the service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Weigh 0.13g of pretreated (activation → sensitization of carbon nanotubes) CNT and add it to 50ml of a solution with a platinum content of 0.128g and a nickel content of 0.08g, and the auxiliary agent is 0.32g of anhydrous Sodium acetate + 0.32g trisodium citrate, ultrasonic vibration for 10 minutes under airtight conditions, and then mechanically stirred for 30 minutes to prepare a raw material solution, sodium hypophosphite as reducing agent, the concentration of sodium hypophosphite under the entire reduction condition is 0.8mol / L, the concentration of sodium hypophosphite is 6 times (molar ratio) of noble metal, and the raw material liquid passes N 2 Under atmospheric conditions, in a water bath at 85-90°C, add a solution of 10ml of reducing agent dropwise under mechanical stirring, adjust the pH value with NaOH concentrated solution during the reduction process, and control the pH value at 9, and complete the reduction after 1 hour under sufficient stirr...
Embodiment 2
[0027]Example 2: Weigh 0.13g of pretreated (CNT activation → sensitization) CNT and add it to 50ml of a solution with a platinum content of 0.128g and a nickel content of 0.039g, and the auxiliary agent is 0.32g of anhydrous sodium acetate +0.32g trisodium citrate, ultrasonically oscillate for 10 minutes under airtight conditions, and then mechanically stir for 30 minutes to prepare a raw material solution. Sodium hypophosphite is used as a reducing agent. The concentration of sodium hypophosphite under the entire reduction condition is 0.8mol / L. The concentration of sodium hypophosphite is 8 times that of noble metal (molar ratio), and the raw material liquid is passed through N 2 Under atmospheric conditions, in a water bath at 85-90°C, add a solution of 10ml of reducing agent drop by drop under mechanical stirring. During the reduction process, use NaOH concentrated solution to adjust the pH value, and the pH value is controlled at 11. After 0.5h under the condition of suffi...
Embodiment 3
[0028] Example 3: Weigh 0.21g of pretreated (CNT activation→sensitization) CNT and add it to 50ml of a solution with a platinum content of 0.279g and a nickel content of 0.04g, and the auxiliary agent is 0.32g of anhydrous sodium acetate + 0.32g trisodium citrate, ultrasonically oscillated for 10 minutes under airtight conditions, and then mechanically stirred for 30 minutes to prepare a raw material solution. Sodium hypophosphite was used as a reducing agent. The concentration of sodium hypophosphite under the entire reduction condition was 1.05mol / L. The concentration of sodium hypophosphite is 8 times that of noble metal (molar ratio), and the raw material liquid is passed through N 2 Under atmospheric conditions, in a water bath at 85-90°C, add a solution of 10ml of reducing agent drop by drop under mechanical stirring. During the reduction process, use NaOH concentrated solution to adjust the pH value, and the pH value is controlled at 11. After 0.5h under the condition of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com