Method for preparing porous titanium dioxide based on light assistance
A porous titanium dioxide and light-assisted technology, applied in the direction of titanium dioxide, titanium oxide/hydroxide, etc., can solve the problems of low yield and poor repeatability, and achieve the effects of small solvent pollution, large pore volume, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 5mL butyl titanate Ti(OC) to 50mL ethylene glycol solvent 4 h 9 ) 4 , after stirring for 30 minutes, the solution was transferred to a round-bottomed flask, refluxed at 160°C for 2 hours, and then naturally cooled to room temperature;
[0033] The above solution is separated by a centrifuge, and then washed repeatedly with absolute ethanol for 3 times to obtain titanium glycolate;
[0034] The resulting titanium glycolate solid was dried at room temperature to obtain titanium glycolate Ti(OCH 2 CH 2 O) 2 Precursor solid powder;
[0035] Weigh 4 grams of titanium glycolate precursor and disperse it in 400 mL of water, and then irradiate the solution with a 400-watt ultraviolet lamp for 2 hours under a nitrogen protective atmosphere to obtain a porous titanium dioxide material.
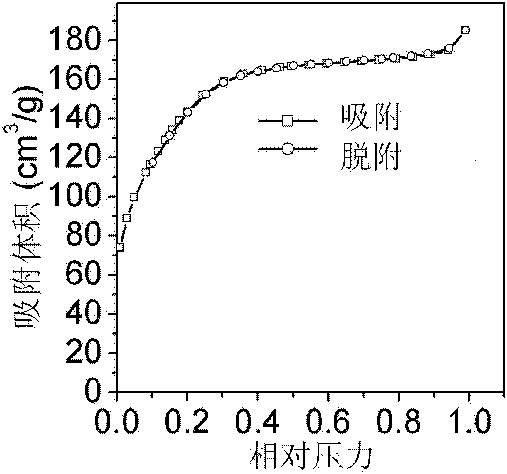
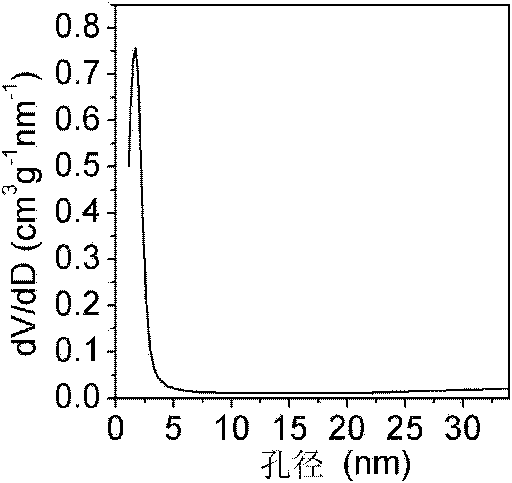
[0036] Some structural characterizations of the porous titania materials prepared by the above method were carried out. figure 1 Shown are the nitrogen adsorption-desorption isotherms ...
Embodiment 2
[0039] Add 5mL butyl titanate Ti(OC) to 50mL ethylene glycol solvent 4 h 9 ) 4 , after stirring for 30 minutes, the solution was transferred to a round-bottomed flask, refluxed at 160°C for 2 hours, and then naturally cooled to room temperature;
[0040] Using a centrifuge to separate the above solution, and then repeatedly washing it with absolute ethanol 3 times to obtain a metal alkoxide solid;
[0041] The obtained metal alkoxide solid is dried at room temperature to obtain titanium glycolate Ti(OCH 2 CH 2 O) 2 Precursor solid powder;
[0042]Weigh 4 grams of titanium glycolate precursor and disperse it in 400mL of water, and then irradiate the solution with a 400-watt UV lamp for 0.25 hours, 0.5 hours, 1.0 hours, 1.5 hours, and 2 hours under a nitrogen protective atmosphere. , sample 10mL at a given time and titrate with potassium dichromate to measure the number of electrons contained in the porous titanium dioxide material.
[0043] Figure 4 Shown is the curve ...
Embodiment 3
[0045] Add 5mL butyl titanate Ti(OC) to 50mL ethylene glycol solvent 4 h 9 ) 4 , after stirring for 30 minutes, the solution was transferred to a round-bottomed flask, refluxed at 160°C for 2 hours, and then naturally cooled to room temperature;
[0046] Use a centrifuge to separate the above solution, and then use absolute ethanol to wash repeatedly 3 times to obtain titanium glycolate;
[0047] The resulting titanium glycolate solid is dried at room temperature to obtain titanium glycolate Ti(OCH 2 CH 2 O) 2 Precursor solid powder;
[0048] Weigh 4 grams of titanium glycolate precursor and disperse it in 400 mL of water, and then irradiate the solution with a 400-watt ultraviolet lamp for 2 hours under a nitrogen protective atmosphere to obtain a porous titanium dioxide material containing electrons. Each gram of titanium dioxide sample contains 1.4 mmol of electrons.
[0049] Weigh 2 grams of the above-mentioned electron-containing titanium dioxide, and add it into 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com