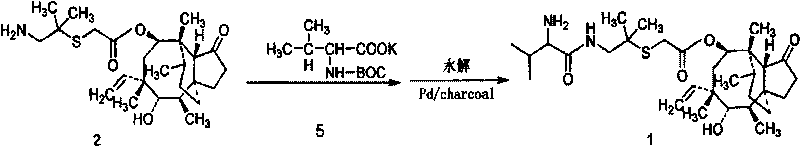Method for synthesizing valnemulin hydrochloride
A technology for the synthesis of warnemulin hydrochloride and its synthesis method, which is applied in the field of synthesis of warnemulin hydrochloride, can solve the problems that it cannot be used as medicine, is not suitable for industrial production, and does not exceed 93%, and achieves loss reduction and volatilization. The effect of reducing the property and increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
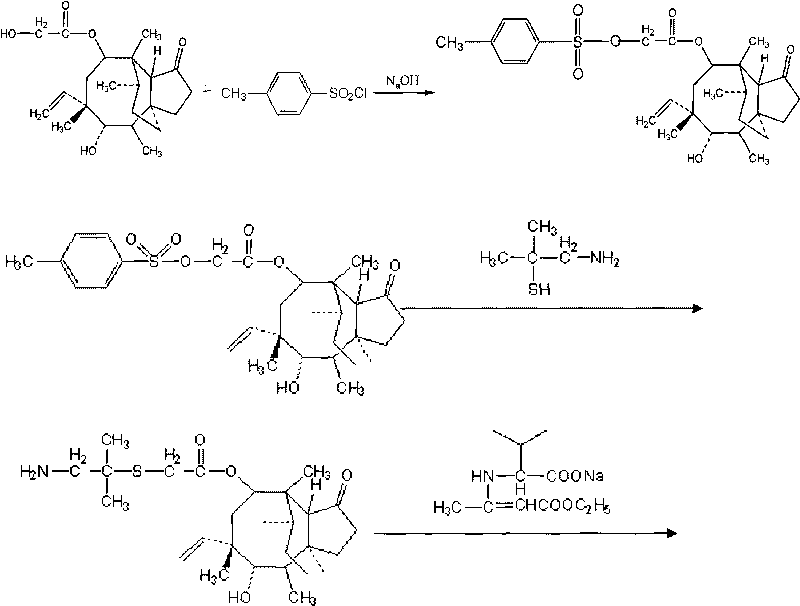
[0044] Add 37.85 g of pleuromutilin and 150 ml of acetone into a 250 ml three-necked flask equipped with a mechanical stirrer and a thermometer, and stir to dissolve it. Place the three-necked bottle in a low-temperature tank and cool down to 0°C. Add 19.06g of p-toluenesulfonyl chloride and 15ml of 40wt% NaOH, and stir the reaction for 20min. After the reaction, the solvent was concentrated to dryness to obtain pleuromutilin.
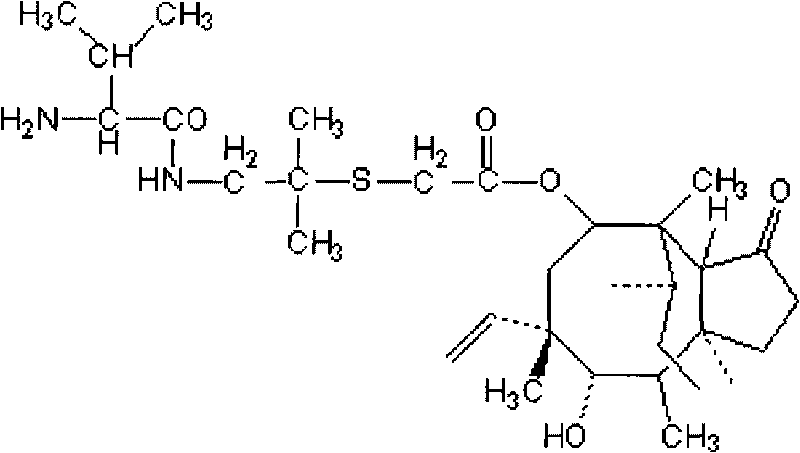
[0045] Add 53.2g pleuromutilin sulfonated, 14.2g dimethylcysteamine hydrochloride, 1.2g tetrabutylammonium bromide, 30ml 10N NaOH solution in a 500ml three-necked flask equipped with mechanical stirring, thermometer and reflux device and 100ml tert-butyl methyl ether. Heat and stir at 53° C. for 1 h, detect by TLC, and add 250 ml of water to the three-neck flask if the reaction is complete. The reaction temperature was lowered to 0°C, and after stirring at this temperature for 30 minutes, a precipitate was found to be formed, filtered, and the filte...
Embodiment 2
[0051] Add 30.1 g of pleuromutilin and 180 ml of ethanol into a 250 ml three-necked flask equipped with a mechanical stirrer and a thermometer, and stir to dissolve it. Place the three-necked bottle in a low-temperature tank and cool down to -5°C. 15.1 g of p-toluenesulfonyl chloride and 12 ml of 40 wt% NaOH were added, and the reaction was stirred for 30 min. After the reaction, the solvent was concentrated to dryness to obtain a solid product.
[0052] Add 100g of pleuromutilin, 32g of dimethylcysteamine hydrochloride, 3g of cetyltrimethylammonium bromide, and 50ml of 10N NaOH into a 500ml three-necked flask equipped with mechanical stirring, a thermometer and a reflux device solution and 300ml 2-methylisoamylone. Heat and stir at 55° C. for 1 h, and detect by TLC. If there are no spots of dimethylcysteamine hydrochloride, the reaction is complete; after the reaction, the organic phase is directly used for the synthesis of warnemulin hydrochloride.
[0053] Add 8g of soli...
Embodiment 3
[0058] Except that butyl acetate is used as solvent when preparing ATAM, others are the same as Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com