Composite method of high-energy insensitive explosive 2,6-diamino-3,5-dinitro pyridine-1-oxide
A technology of dinitropyridine and a synthesis method, applied in the field of explosive synthesis, can solve the problems of difficulty in industrialization, multiple impurities, high cost, etc., and achieve the effects of simple product post-processing, simple process, and strong market competitiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
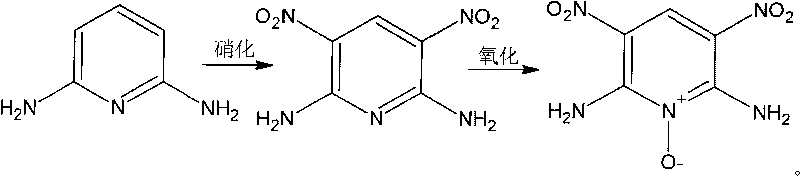
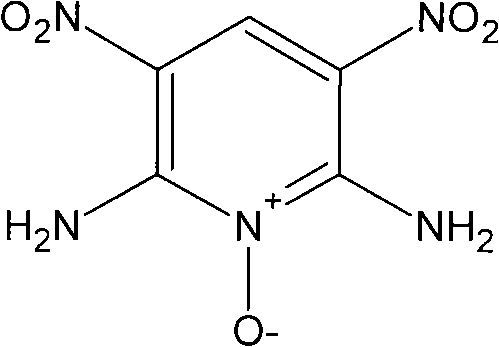
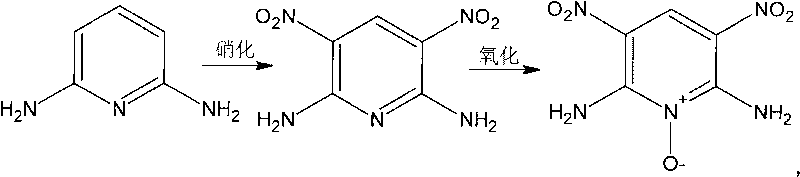
[0032] For the synthesis of 2,6-diamino-3,5-dinitropyridine, see accompanying drawing II for the synthetic route. In a 100mL three-neck flask equipped with a stirrer and a thermometer, add 75mL of 98% concentrated H 2 SO 4 , add 2.18g 2,6-diaminopyridine under stirring, after the solid is completely dissolved, continue to stir until the temperature of the mixture drops below 30°C; add 0.075mol KNO within 10min 3 (Ice-water bath controls the temperature of the mixture below 30°C), adding KNO 3 Keep stirring vigorously during the process; after the reaction is over for three hours, pour the mixture into crushed ice, stir, and a large amount of tan solid precipitates, filter and wash with water; transfer it to a 100mL flask after vacuum drying, add 50mL 2molL -1 NaOH, stirred for 2 hours, cooled, filtered, washed with water, dried, the obtained solid was boiled in distilled water for one hour, filtered, dried, weighed, and the yield was 75%.
Embodiment 2
[0034] For the synthesis of 2,6-diamino-3,5-dinitropyridine, see accompanying drawing II for the synthetic route. In a 100mL three-neck flask equipped with a stirrer and a thermometer, add 15ml of 65% fuming H 2 SO 4 , add 2.18g 2,6-diaminopyridine under stirring, after the solid is completely dissolved, continue to stir until the temperature of the mixture drops below 20°C; add 0.04mol NaNO within 10min 3 (Ice-water bath controls the temperature of the mixture below 20°C), maintain vigorous stirring during the addition of nitrate; after the reaction for three hours, pour the mixture into crushed ice, stir, and precipitate a large amount of yellow-brown solid, filter, wash with water; vacuum dry Then transfer to a 100ml flask, add 50ml2molL -1 NaOH, stirred for 2 hours, cooled, filtered, washed with water, and dried. The resulting solid was boiled in distilled water for one hour, filtered, and dried. The yield was 90%.
Embodiment 3
[0036] For the synthesis of 2,6-diamino-3,5-dinitropyridine, see accompanying drawing II for the synthetic route. In a 5L three-necked flask equipped with a stirrer and a thermometer, add 2400ml of 20% fuming sulfuric acid, add 300g of 2,6-diaminopyridine under stirring, and continue stirring until the temperature of the mixture drops below 30°C after the solid is completely dissolved; Add 240ml fuming HNO within 15min 3 (Ice-water bath controls the temperature of the mixture below 30°C), add fuming HNO 3 Keep stirring vigorously during the process; after the reaction for three hours, pour the mixture into crushed ice, stir, and a large amount of yellow-brown solid precipitates, filter and wash with water; after vacuum drying, transfer into 5000ml2molL -1 The NaOH solution was stirred for 2 hours, cooled, filtered, washed with water, and dried. The resulting solid was boiled in distilled water for one hour, filtered, and dried. The yield was 93%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com