Human papilloma virus (HPV) fusion protein, gene, carrier, strain, preparation method and application
A fusion protein and strain technology, applied in the field of bioengineering, can solve the problems that virus particles are unlikely to develop into vaccines, HPV is difficult to cultivate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Design and synthesis of human papillomavirus type 16 L2E7 fusion protein Escherichia coli expression optimization codon gene
[0052] The HPV16 late gene L2 and early gene E7 isolated and cloned from cervical cancer patient specimens were sequenced, and according to the obtained HPV16 late gene L2 and early gene E7 encoded polypeptide amino acid sequences, the E7 protein was combined with the two binding sites of pRB The codons of the key amino acids cysteine at position 24 and glutamic acid at position 26 were mutated to glycine codons GGT and GGG, respectively, to eliminate their tumor transformation activity. The minor capsid protein sequence L2 of the HPV16 virus is combined with the E7 protein sequence of the HPV16 virus to be designed as a fusion protein as follows:
[0053] MRHKRSAKRTKRASATQLYKTCKQAGTCPPDIIPKVEGKTIADQ
[0054] ILQYGSMGVFFGGLGIGTGSGTGGRTGYIPLGTRPPTATDTLAPV
[0055] RPPLTVDPVGPSDPSIVSLVEETSFIDAGAPTSVPSPSIPPDVSGFSIT
[0056] TSTD...
Embodiment 2
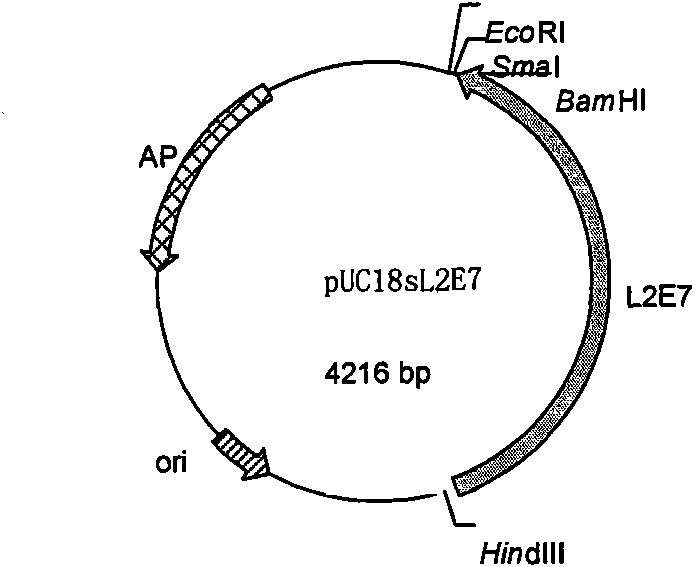
[0109] The above sL2E7 nucleotide sequence was sent to Gene Synthesis Company for synthesis, and the company cloned it into pUC18 to obtain the recombinant cloning plasmid pUC18sL2E7 of the synthetic gene. For the structure of the recombinant cloning plasmid, see figure 1 .
[0110] The pUC18 used in this example is a commercially available plasmid vector, and its specific structure can be found in the literature: Sambrook J, Fritsch EF, and Manny Artis T, Molecular Cloning Experiment Guide. 1992. Second Edition (Beijing): Science Press: Page 9.
Embodiment 3
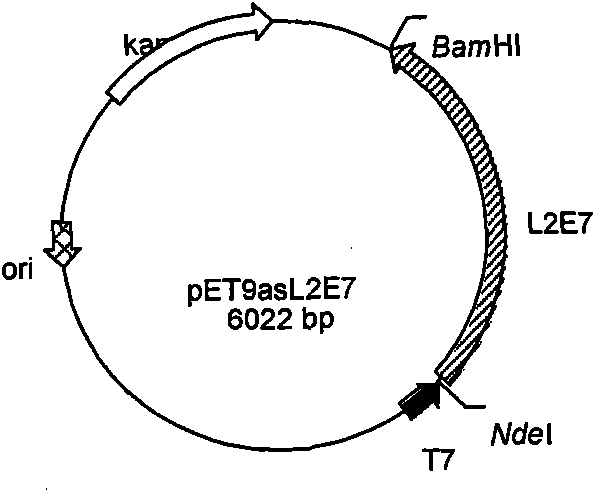
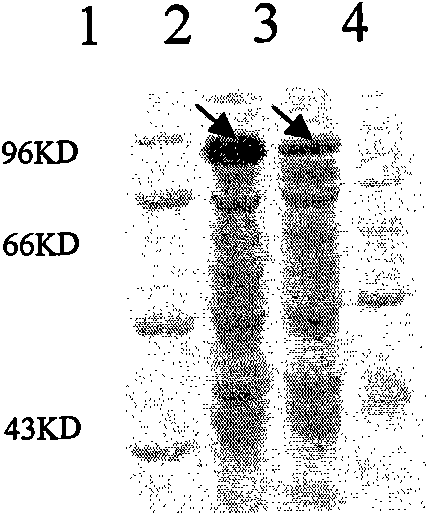
[0112] The synthesized and cloned L2E7 optimized gene was digested by enzyme digestion. The operation of the enzyme digestion and digestion was as follows: first digested with Nde I enzyme in a 37°C water bath for 2 hours, then recovered with an agarose gel recovery kit, and then BamH I enzyme 37 Digest in water bath at ℃ for 2 hours, and finally recover the fragments with an agarose gel recovery kit. The Nde I and BamH I enzymes used are Biolabs products and purchased from Beijing North Yitao Trading Co., Ltd.; Agarose gel recovery kit It is a product of Beijing Tiangen Biochemical Technology Co., Ltd., and then inserted into the Nde I and BamH I sites of the Escherichia coli expression vector pET9a. The prokaryotic expression recombinant plasmid pET9asL2E7 with correct insertion was obtained after Nde I and BamH I enzyme digestion and sequencing screening , its structure diagram is as follows figure 2 shown. The pET9a used is a commercially available expression plasmid vec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com