Nanoparticle probes for detecting ribosome inactivating protein, manufacturing method thereof and use thereof
A ribosome inactivation and nanoparticle technology is applied in the field of nanoparticle probes and their preparation, which can solve the problems of complicated steps and low sensitivity, and achieve the effects of simple development process, high sensitivity, and easy popularization and application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
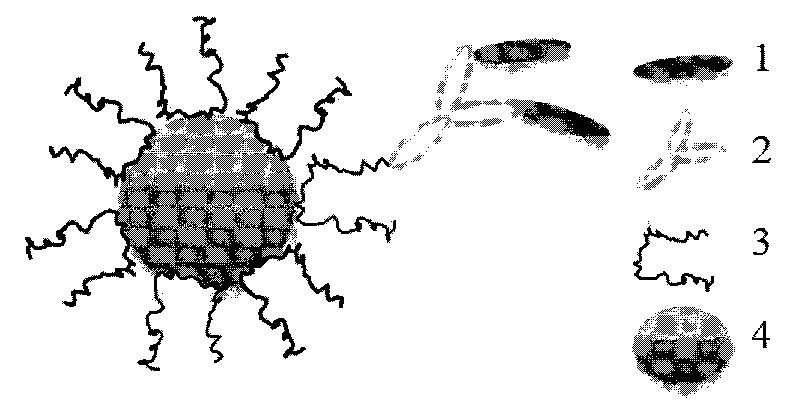
[0057] ③ Preparation of nanoparticle probes
[0058] The antibody modified by the binding site is reacted with the surface-modified matrix nanoparticles at a constant temperature, the reaction temperature is 20-50°C, the reaction time is 0.5-5h, and the pH value is between 7.0-8.5, and the specific antibody is obtained. nanoparticle probes.
[0059] ④ Separation of nanoparticle probes
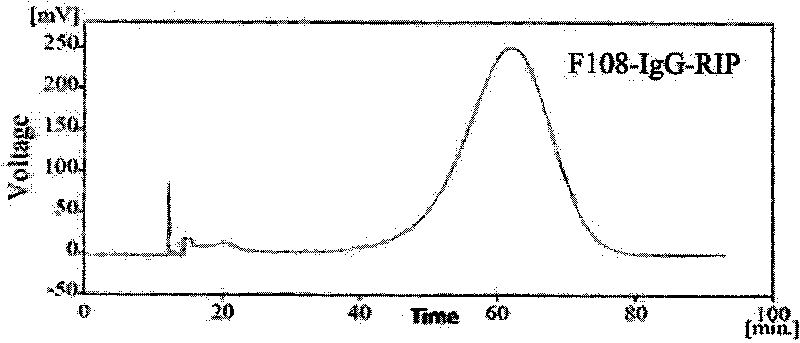
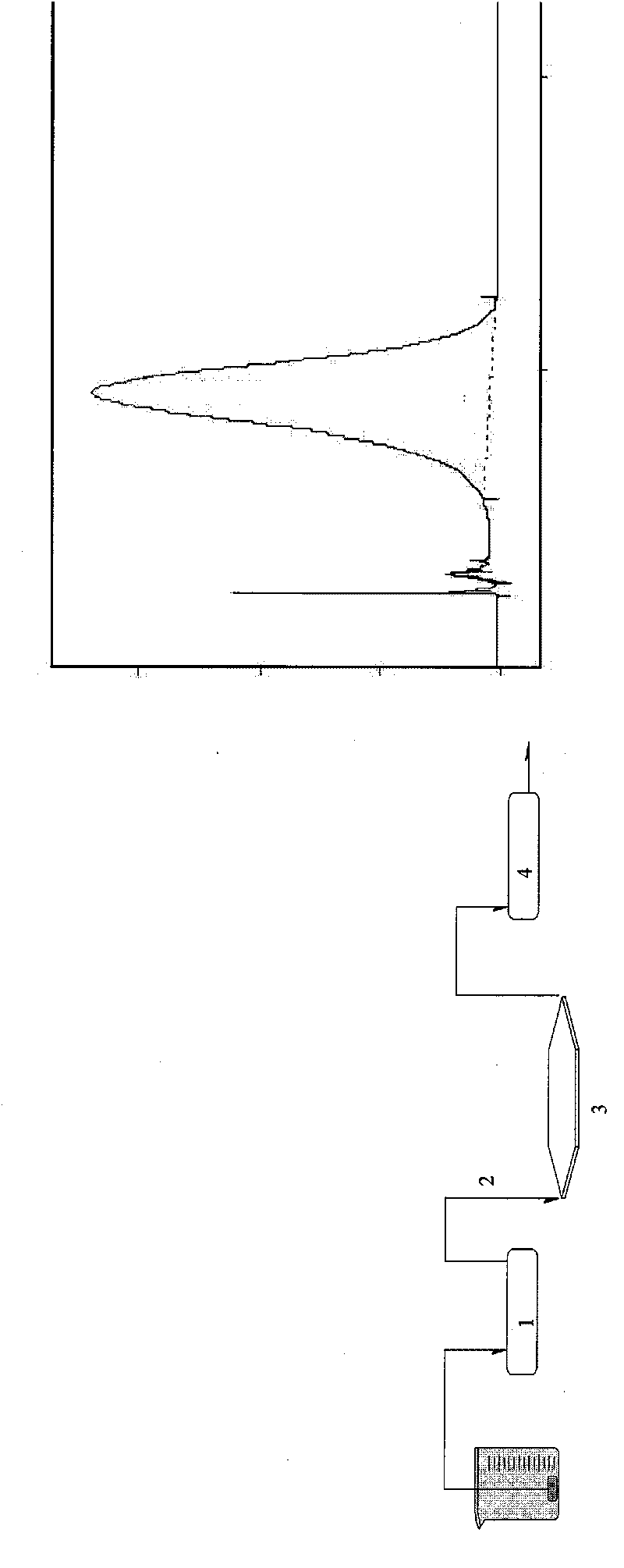
[0060] Using sedimentation field flow separation technology, it can separate suspended particles in the range of 20nm-1μm in the fluid. Using the mobile phase containing FL-70 surfactant, under the action of external fields such as centrifugal force field, according to the difference in particle size and quality, Nanoparticles of different particle sizes are distributed in a certain way in the thickness of the channel, and the fluid near the channel wall will flow slower than in the center; the difference in the flow velocity of nanoparticles of different particle sizes in the channel will lea...
Embodiment 1
[0085] Nanoparticle probe and its preparation for detection of ricin
[0086] ① Surface modification of polystyrene matrix nanoparticles by modifier F108-PDS
[0087] Proportionally weigh a certain volume of modifier F108-PDS (0.01-10g / L) and add it to the polystyrene nanoparticle suspension with a particle size of 290nm. Modifiers, and then centrifuge under certain conditions to separate the modifiers that are not bound or not tightly bound, discard the supernatant and re-dilute the nanoparticles with phosphate buffer.
[0088] ②Chemical modification of mouse anti-human IgG monoclonal antibody sites
[0089] Use n-hydroxysuccinimide 3-(2-pyridyldimercapto) propionate (concentration: 0.01-5g / L) to modify the active site of the antibody, and weigh them respectively according to the ratio of the two (0.1-1.5). Take a certain volume (1-20 μL) of the modifier and add it to the antibody solution. After reacting at a temperature of 20-50°C, use a specific chromatographic column t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com