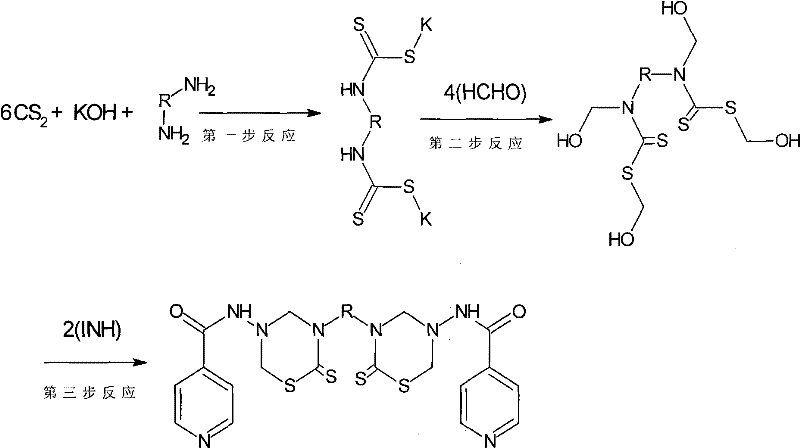
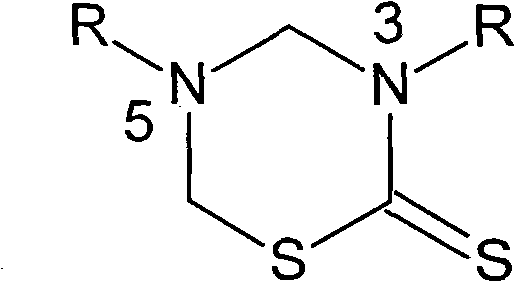
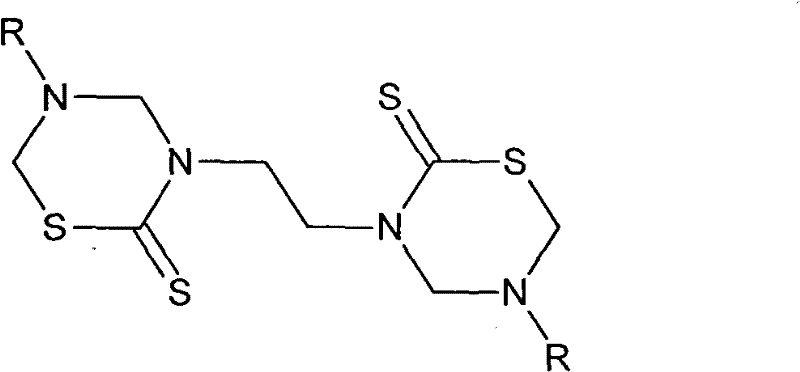
Isoniazid thiadiazine thione derivative
A technology of thiadiazinethione and isoniazid, which is applied in the direction of organic chemistry, organic active ingredients, antibacterial drugs, etc., can solve the problems of ineffective delivery of drugs, achieve effective anti-tuberculosis, reduce drug resistance, and good The effect of antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Synthesis of N,N'-[ethane-1,2-diyl(6-thio-1,3,5-thiadiazine-5,3-diyl)]bisisonicotinamide, structure diagram of the compound as follows:
[0073]
[0074] Figure 7N, N'-[ethane-1,2-bisyl (6-thio-1,3,5-thiadiazine-5,3-diyl)] bisisonicotinamide
[0075] The chemical name of this product is: N, N'-[ethane-1,2-diylbis(6-thioxo-1,3,5-thiadiazinane-5,3-diyl)]diisonicotinamide, and the molecular formula is C 20 h 22 N 8 S 4 o 2 , referred to as ETTD. The molecular weight is 534. The specific synthesis steps are as follows: put 1.5g (25mmol) ethylenediamine in a flask, add 75mL of toluene, heat to dissolve at 35°C, add 7mL (20%, 25mmol) KOH solution and stir for about 2 minutes, then drop 12mL (200mmol) ) carbon disulfide, reacted at 35°C for 8 hours, cooled to room temperature, added 13.6mL (150mmol) formaldehyde, and continued to stir for 3 hours. 6.86g (50mmol) of isoniazid was dissolved in 12.5mL of pH7.8 phosphate buffer solution and 25mL of absolute ethanol, dro...
Embodiment 2
[0077] Synthesis of N,N'-[butane-1,4-diyl(6-thio-1,3,5-thiadiazine-5,3-diyl)]bisisonicotinamide, structure diagram of the compound as follows:
[0078]
[0079] Figure 8N, N'-[butane-1,4-bisyl (6-thio-1,3,5-thiadiazine-5,3-diyl)] bisisonicotinamide
[0080] The chemical name of this product is: N, N'-[butane-1,4-diylbis(6-thioxo-1,3,5-thiadiazinane-5,3-diyl)]diisonicotinamide, and the molecular formula is C 22 h 26 N 8 S 4 o 2 , referred to as BTTD. The molecular weight is 562. The specific synthesis steps are as follows: put 2.2g (25mmol) of butanediamine in a flask, add 75mL of toluene, heat and dissolve at 35°C, add 7mL (20%, 25mmol) of KOH solution and stir for about 2 minutes, then drop 12mL (200mmol) of ) carbon disulfide, reacted at 35°C for 8 hours, cooled to room temperature, added 13.6mL (150mmol) formaldehyde, and continued to stir for 3 hours. 6.86g (50mmol) of isoniazid was dissolved in 12.5mL of pH7.8 phosphate buffer solution and 25mL of absolute etha...
Embodiment 3
[0082] Synthesis of N,N'-[butane-1,3-diyl(6-thio-1,3,5-thiadiazine-5,3-diyl)]bisisonicotinamide, structure diagram of the compound as follows:
[0083]
[0084] Figure 9N, N'-[isobutane-1,3-bisyl (6-thio-1,3,5-thiadiazine-5,3-diyl)] bisisonicotinamide
[0085] The chemical name of this product is: N,N'-[isobutane-1,12-diylbis(6-thioxo-1,3,5-thiadiazinane-5,3-diyl)]diisonicotinamide, the molecular formula is C 22 h 26 N 8 S 4 o 2 , referred to as ITTD. The molecular weight is 562. The specific synthesis steps are as follows: put 2.2g (25mmol) of isobutylene diamine in a flask, add 75mL of toluene, heat to dissolve at 35°C, add 7mL (20%, 25mmol) of KOH solution and stir for about 2 minutes, then drop into 12mL ( 200mmol) of carbon disulfide, reacted at 35°C for 8 hours, cooled to room temperature, added 13.6mL (150mmol) of formaldehyde, and continued to stir for 3 hours. 6.86g (50mmol) of isoniazid was dissolved in 12.5mL of pH7.8 phosphate buffer solution and 25mL of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com