Novel preparation method for sitafloxacin intermediate
A sitafloxacin and intermediate technology, applied in the new preparation field, can solve the problems of high cost, high equipment requirements, harsh reaction conditions, etc., and achieves mild reaction conditions, reduced splitting costs, and low equipment requirements. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
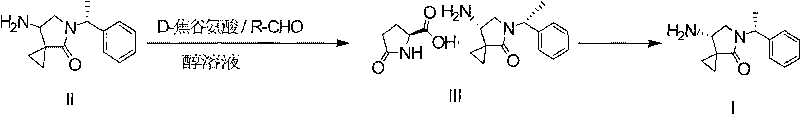
[0046] Example 1: (7S)-amino-5-[1(R)-5-phenylethyl]4-oxo-5-azaspiro[2.4]-heptane pyroglutamate (formula III) preparation
[0047] The racemate (7R, 7S)-amino-5-[1(R)-5-phenethyl]4-oxo-5-azaspiro[2.4]-heptane (Formula II) 23.0g (100mmol ) into a 2000ml reaction flask, 1000ml of 80% isopropanol solution was added, the temperature was raised to 50°C, 10.3g (80mmol, 0.8 equivalent) of pyroglutamic acid was added, and the temperature was controlled at 60°C for 5 hours. After 5 hours, 0.95 g (5 mmol, 5%) of 3,4-dichlorosalicylaldehyde was added, and the temperature was controlled at 80° C. for 15 hours. After cooling down to room temperature, let stand for 12 hours, filter, and dry under reduced pressure at 60°C to obtain 28.9 g of the compound of formula III with a yield of 81%.
Embodiment 2
[0048] Example 2: Preparation of (7S)-amino-5-[1(R)-5-phenylethyl]4-oxo-5-azaspiro[2.4]-heptane (Formula I)
[0049] (7S)-amino-5-[1(R)-5-phenylethyl]4-oxo-5-azaspiro[2.4]-heptane pyroglutamate (Formula III) 28.6g (80mmol ) was dissolved in 500ml of water, and the pH was adjusted to about 12 with 1mol / L sodium hydroxide solution, and the solid was precipitated, filtered, and dried to obtain (7S)-amino-5-[1(R)-5-phenylethyl]4- Oxo-5-azaspiro[2.4]-heptane (Formula I) 18.1g, yield 97%,
[0050] 1 HNMR (400mz, CDCl3) δ 0.8-1.42 (m, 4H), 1.53 (d, 1H, J=7Hz), 2.88 (dd, 1H, J=10.2Hz). 3.3-3.9(m, 2H), 4.28(brs, 1H), 5.24(q, 1H, J-7Hz), 7.29(m, 5H)
[0051] Purity determination: CHIRALPAK MA (+) chiral analysis column, n-hexane: isopropanol: methanol = 60:30:10 (0.1% ethylenediamine), 0.6mL / min, UV254nm, purity > 97%, ee > 95 %.
Embodiment 3
[0052] Example 3: (7S)-amino-5-[1(R)-5-phenylethyl]4-oxo-5-azaspiro[2.4]-heptane pyroglutamate (formula III) preparation
[0053] The racemate (7R, 7S)-amino-5-[1(R)-5-phenethyl]4-oxo-5-azaspiro[2.4]-heptane (Formula II) 23.0g (100mmol ) into a 2000ml reaction flask, 1000ml of 80% propanol solution was added, the temperature was raised to 50°C, 10.3g (80mmol, 0.8 equivalent) of pyroglutamic acid was added, and the temperature was controlled at 60°C for 5 hours. After 5 hours, 0.83 g (5 mmol, 5%) of 5-nitrosalicylaldehyde was added, and the temperature was controlled at 80° C. for 10 hours. After cooling down to room temperature, let it stand for 15 hours, filter, and dry under reduced pressure at 60°C to obtain 28.1 g of the compound of formula III with a yield of 78.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com