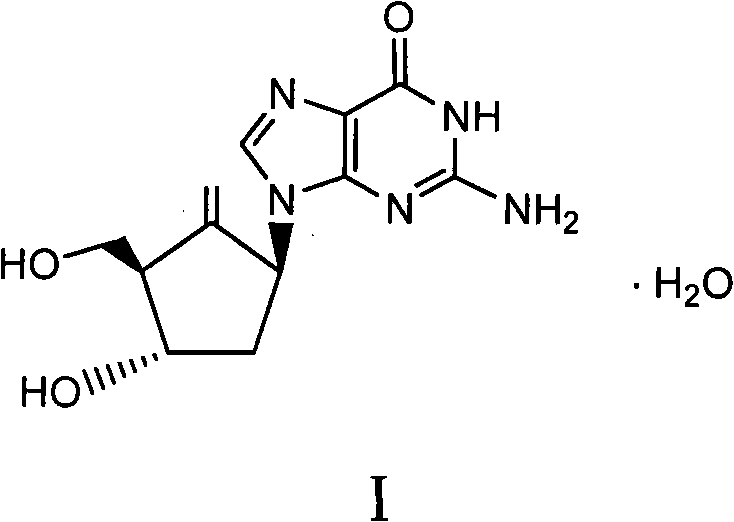
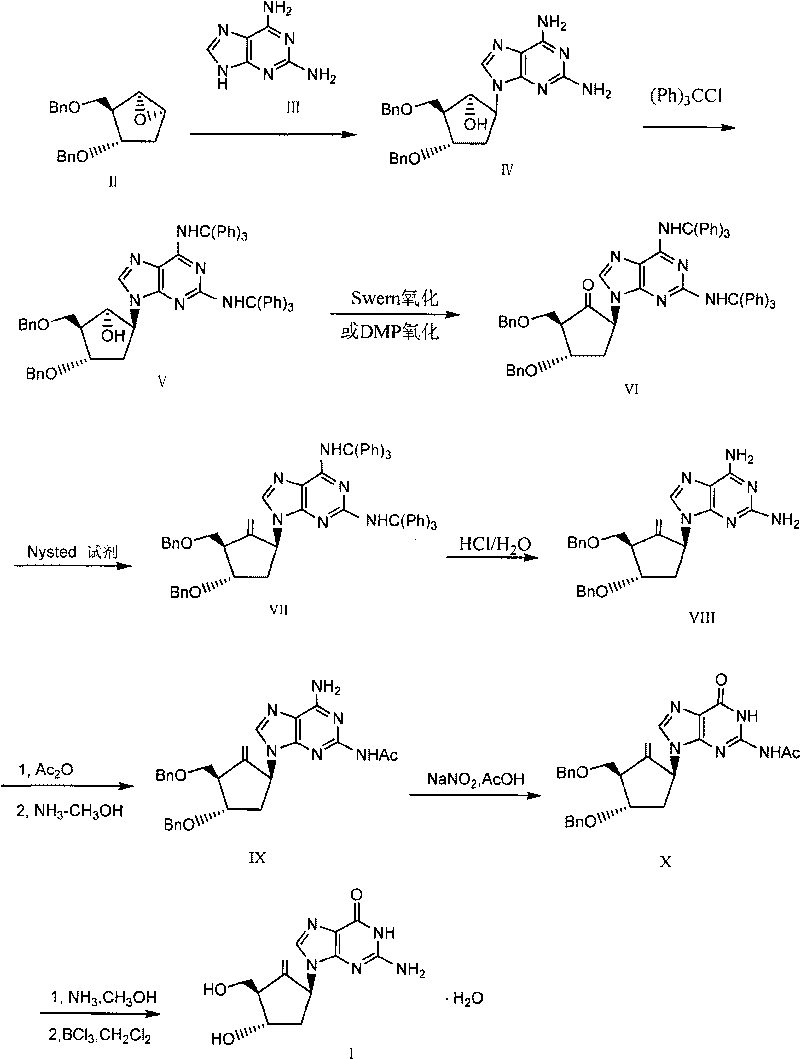
Method for preparing entecavir
A technology of entecavir and benzyloxy, which is applied in the field of preparation of entecavir, a drug for treating hepatitis B, to achieve the effects of easy separation and purification, simple reaction technical conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of [1S(1α, 2β, 3α, 5β)]-5-(2,6-diaminopurin-9-yl)-3-benzyloxy-2-benzyloxymethylcyclopentanol IV
[0028] 15g of 2,6-diaminopurine, 2g of 60% NaH, and 250ml of dry DMF were mixed, stirred at 85°C for 20 minutes, cooled to room temperature, and added [1S(1α,2α,3β,5α)]-3-Benzyloxy- 15.5 g of 2-benzyloxymethyl-6-oxabicyclo[3,1,0]hexane II and 61.4 g of 18-crown ether were heated under reflux for four hours. After the reaction, the DMF was evaporated under reduced pressure, and the residue was extracted with ethyl acetate, washed with water and saturated sodium chloride solution in turn, dried, concentrated, and the crude product was passed through the column to obtain 17.2 g of condensate IV, with a yield of 74%.
Embodiment 2
[0030] [1S(1α, 2β, 3α, 5β)-5-[2,6-bis(tritylamino)purin-9-yl]-3-benzyloxy-2-benzyloxymethylcyclopentanol Preparation of V
[0031] Mix 12g of condensate IV obtained in the previous step, 21.76g of triphenylchloromethane, 7.9g of triethylamine, and 240ml of dry dichloromethane, and stir overnight at room temperature. After the reaction was completed, saturated sodium bicarbonate solution was added to terminate the reaction. Separate the organic layer, extract the aqueous layer with dichloromethane, combine the organic layers, wash with water and saturated sodium chloride solution successively, dry, evaporate the solvent and recrystallize to obtain 15 g of the double-protected product V with a yield of 60%.
Embodiment 3
[0033] [2R(2α, 3β, 5α)]-5-[2,6-bis(tritylamino)purin-9-yl]-3-benzyloxy-2-benzyloxymethylcyclopentanone VI preparation of
[0034] After mixing 60ml of anhydrous dichloromethane and 4.17g of oxalyl chloride, add dropwise a solution of 5g of dimethyl sulfoxide in 20ml of anhydrous dichloromethane at -70°C, stir for 15 minutes, then add dropwise the solution prepared in Example 2 A solution prepared with 10 g of the obtained double-protected product V and 40 ml of anhydrous dichloromethane. Continue to react at low temperature for two hours. 7.5 g of triethylamine was added dropwise, followed by stirring for another hour. The reaction solution was poured into saturated sodium bicarbonate solution to terminate the reaction. Separate the organic layer, extract the aqueous layer with dichloromethane, combine the organic phases, wash with water until neutral, dry and evaporate the solvent under reduced pressure to obtain 10 g of crude VI.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com