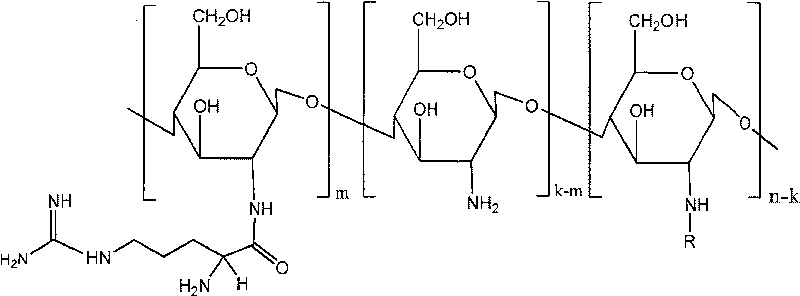
Novel chitosan derivative simulating the structure of cell-penetrating peptide
A kind of technology of chitosan derivative and penetrating peptide, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation of chitosan modified by arginine and phenylalanine (N-arginine-N-phenylalanine chitosan)
[0026] Dissolve chitosan (Mw20000) (2g) in (150ml) 2% acetic acid solution, stir until completely dissolved, about 24h, add a certain amount of arginine (0.98g) protected by BOC anhydride, BOC anhydride protected Phenylalanine (1.25g), NHS (1.35g) and EDC (5.58g), stirred for 48h, added 2ml of trifluoroacetic acid, stirred at room temperature for 24h, dialyzed with dialysis bag (MWCO 3500), freeze-dried, after drying, 2.2 g of a water-soluble light yellow powder was obtained.
[0027] FTIR: 1615.97 (arginine guanidine group), 1526.23, 1563.58 (amide bond formed by arginine and phenylalanine), 3086.12, 1532.31, 701.59 (phenyl ring of phenylalanine).
[0028] The degree of substitution of arginine and the degree of substitution of phenylalanine are 17% as measured by elemental analysis combined with nuclear magnetic resonance.
Embodiment 2
[0030] Membrane Penetrating Ability Experiment of N-Arginine-N-Phenylalanine Chitosan
[0031] Compared with chitosan (Mw20000), it shows the membrane-penetrating ability of N-arginine-N-phenylalanine chitosan; N-arginine-N-phenylalanine chitosan is based on the Prepared in Example 1, both chitosan and N-arginine-N-phenylalanine chitosan were labeled with FITC fluorescein.
[0032] ECV-304 was cultured in RPMI 1640 medium containing 10% calf serum, 100 mg / L each of ampicillin and streptomycin. at 37°C, 5% CO 2 , cultured normally under fully saturated humidity, and subcultured once every 3 days.
[0033] Take a ninety-six-well plate, add coverslips, and inoculate ECV-304 cells at a density of 2×10 5cells / well, cultivated overnight, after the cells adhered to the wall, replace the fresh culture medium, remove the culture medium after 30min, and replace with chitosan or N-arginine-N-phenylalanine chitosan containing the same concentration 1mL of 1640 medium (no serum, no dou...
Embodiment 3
[0036] N-Arginine-N-Phenylalanine Chitosan Promotes Gastrointestinal Absorption of Insulin
[0037] Preparation of insulin-N-arginine-N-phenylalanine solution: 10 mg of insulin (potency 29.5 IU / mg, the same below) was dissolved with a small amount of dilute hydrochloric acid, and 10 mg of N-arginine prepared according to Example 1 was added Acid-N-phenylalanine, adjust the pH to 7.4 with 0.1 mol / L NaOH, and dilute to 50 mL with PBS buffer at pH 7.4.
[0038] Preparation of insulin solution for intragastric administration: Dissolve 10 mg of insulin with a small amount of dilute hydrochloric acid, adjust the pH to 7.4 with 0.1 mol / L NaOH, and dilute to 50 mL with PBS buffer with pH 7.4.
[0039] Preparation of insulin solution for subcutaneous injection: Dissolve 10 mg of insulin with a small amount of dilute hydrochloric acid, adjust the pH to 7.4 with 0.1 mol / L NaOH, and adjust the volume to 250 mL with PBS buffer with pH 7.4.
[0040] The SD diabetic rats were randomly divid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com