Method for enhancing stability of nano gold and biological detection method adopting the same
A biological detection and nano-gold technology, which is applied in the direction of analyzing materials through chemical reactions and material analysis through observing the impact on chemical indicators, etc., can solve the cumbersome experimental steps and the stability of double-stranded DNA and single-stranded DNA The difference is not very large, the stability effect and other issues, to achieve the effect of improving the range and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: Preparation of unmodified gold nanoparticles and improvement of the stability of gold nanoparticles by dNMP.
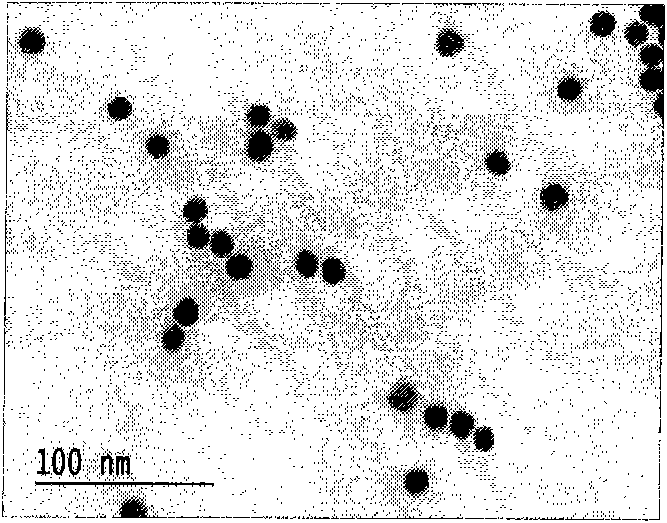
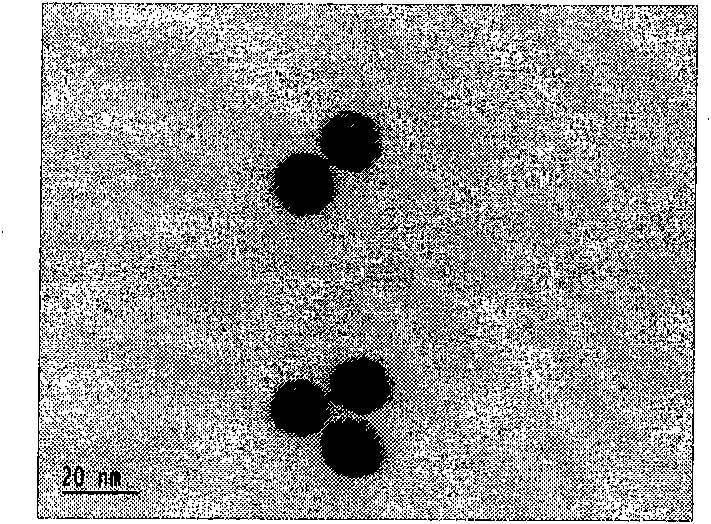
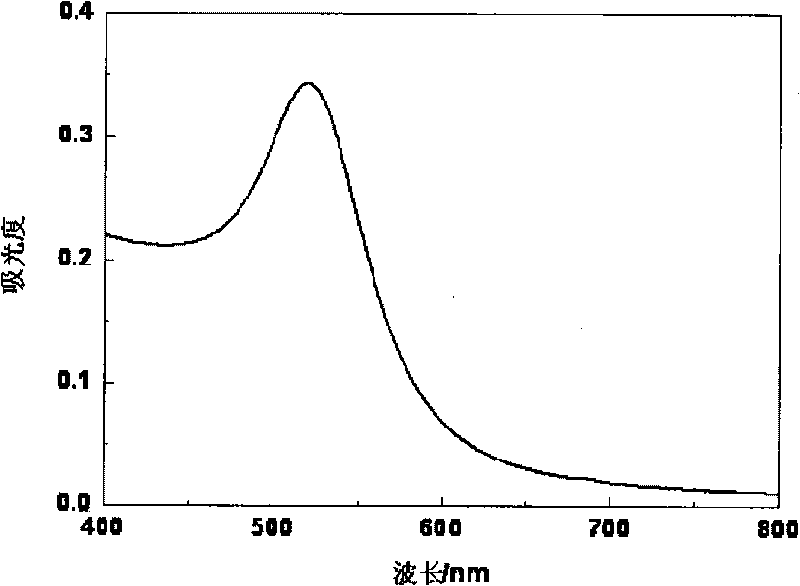
[0055] An aqueous solution of trisodium citrate (25ml, 38.8mM) was quickly added to a boiling solution of auric acid HAuCI4 (250ml, 1mM). After a few minutes, the color of the solution changed from light yellow to dark red. The solution was stirred at reflux for 15 minutes to complete the reaction. Then cool slowly to room temperature. 4 degrees save. According to the ultraviolet absorption intensity of nano gold at 520nm (Fig. 1(C)), the concentration of prepared nano gold is 12.7nM, and the size is 13nm (see Fig. 1(A) and (B).
[0056] In the stability experiment of nano-gold, the concentration of nano-gold is 3nM, 100 μL. dNMP is a mixed solution of dAMP, dTMP, dGMP and dCMP (100μM or / 1mM), in which the ratio of each single nucleotide is the same as that of single-stranded DNA (5'CCGCAAATTGTTC). The amount of 0.6M NaCl phosphate buffer in Ta...
Embodiment 2
[0059] Example 2: Differences in the stabilizing effects of dNMP, single-stranded DNA and double-stranded DNA on unmodified gold nanoparticles
[0060] Double-stranded DNA sample PM / A: 0.25 μl 100 μM 5-terminal phosphorylated single-stranded DNA probe A (5'CCGCAAGACCGCTAGC), 0.25 μl 100 μM target DNA PM (GCTAGCGGTCTTGCGG) fully complementary to the single-stranded DNA probe, 1 microliter of buffer (0.6M NaCl, 10 mM phosphate, pH 7.4), and 0.5 microliter of water were kept at room temperature for 10 minutes.
[0061] Single-stranded DNA sample PM: 0.25 μl of 100 μM target DNA PM (GCTAGCGGTCTTGCGG) that is fully complementary to the single-stranded DNA probe, 1 μl of buffer (0.6M NaCl, 10 mM phosphate, pH 7.4), and 0.75 μl of The water was kept at room temperature for 10 minutes.
[0062] ssDNA sample A: 0.25 µl 100 µM 5-terminal phosphorylated ssDNA probe A (5'CCGCAAGACCGCTAGC), 1 µl buffer (0.6M NaCl, 10 mM phosphate, pH 7.4), and 0.75 µl of water at room temperature for 10 ...
Embodiment 3
[0065] Example 3: Detection of the activity of lambda exonuclease by enhancing the stability of gold nanoparticles
[0066] 10 microliters of enzyme reaction solution contains 5-terminal phosphorylated single-stranded DNA probe A (100pmole, 10μM), (5'PO 4 -CCGCAAGACCGCTAGC) target DNA PM (100pmole, 10μM) fully complementary to the single-stranded DNA probe (GCTAGCGGTCTTGCGG), reaction buffer (67mM Glycine-KOH, 2.5mMMgCI2, 50μg / ml BSA, pH 9.4), and different amounts of λ Exonuclease (from 1unit to 0.1unit). The reaction was carried out at 37°C for 1 hour, followed by heating at 75°C for 10 minutes to inactivate the enzyme. Then the reaction solution was mixed with 200 μl of gold nanoparticles (12.7 nM), and 100 μl of 0.2 M NaCl phosphate solution was added. UV-Vis absorbance measurements were performed and peak shifts were used to quantify color changes and enzyme activity.
[0067] The selectivity of the lambda exonuclease to the substrate can also be performed according to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com