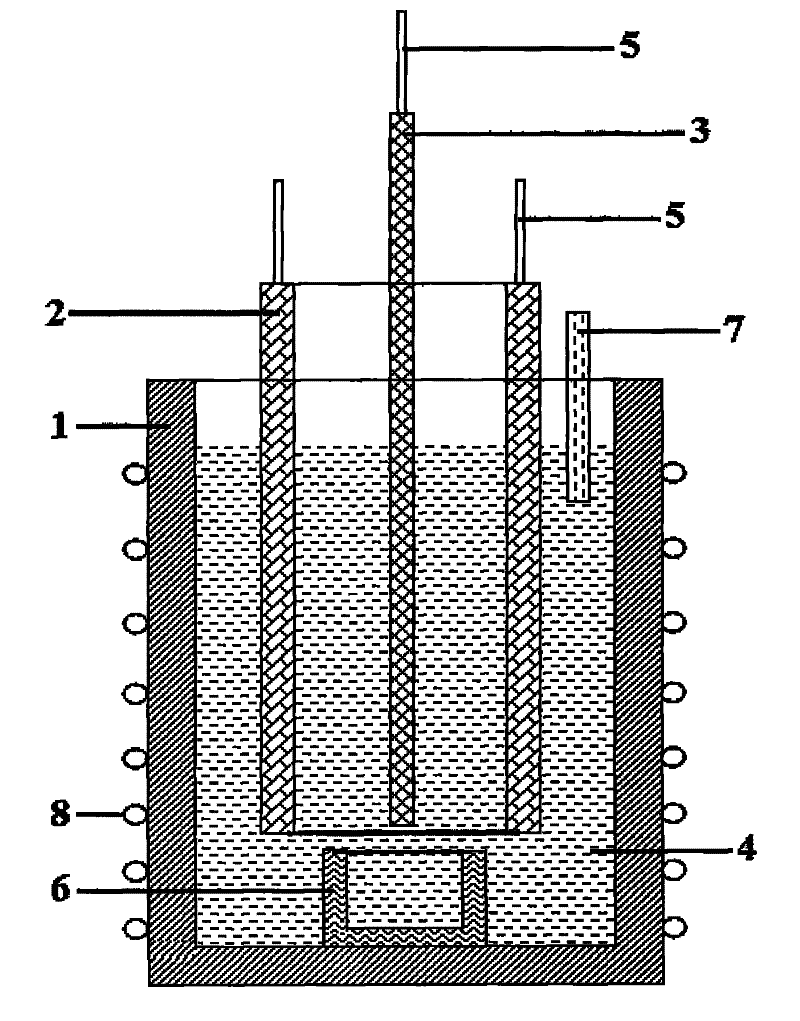
Method for preparing magnesium-nickel alloy by molten salt electrolysis and device therefor
A molten salt, magnesium-nickel technology, applied in the field of nickel alloys, achieves the effects of large magnesium loss, high vacuum requirements and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The steps of the method for preparing the magnesium-nickel alloy are as follows.
[0026] Sodium fluoride and magnesium fluoride are mixed as molten salt, sodium fluoride accounts for 80% of the total mass of the molten salt, and magnesium fluoride accounts for 20% of the total mass of the molten salt.
[0027] Add magnesium oxide accounting for 3.5% of the total mass of sodium fluoride and magnesium fluoride into the molten salt and mix evenly.
[0028] Put the molten salt containing magnesium oxide in the electrolytic cell, heat the molten salt in the electrolytic cell to 1050°C, the two poles start to be energized, and the anode current density during electrolysis is 0.75A / cm 2 , the electrolysis time was 0.9h; then the corundum crucible and cathode filled with magnesium-nickel alloy at the bottom of the electrolytic cell were taken out, put into a new cathode and corundum crucible, and after chemical analysis, a magnesium-nickel alloy with a metal magnesium mass con...
Embodiment 2
[0031] The steps of the method for preparing the magnesium-nickel alloy are as follows.
[0032] Sodium fluoride and magnesium fluoride are mixed as molten salt, sodium fluoride accounts for 95% of the total mass of the molten salt, and magnesium fluoride accounts for 5% of the total mass of the molten salt.
[0033] Add magnesium oxide accounting for 4% of the total mass of sodium fluoride and magnesium fluoride into the molten salt and mix evenly.
[0034] Put the molten salt containing magnesium oxide in the electrolytic cell, heat the molten salt in the electrolytic cell to 1200°C, the two poles start to be energized, and the anode current density during electrolysis is 1A / cm 2 , the electrolysis time was 1.5h; then the corundum crucible and cathode filled with magnesium-nickel alloy at the bottom of the electrolytic cell were taken out, and put into a new cathode and corundum crucible. After chemical analysis, a magnesium-nickel alloy with a metal magnesium mass content o...
Embodiment 3
[0037] The steps of the method for preparing the magnesium-nickel alloy are as follows.
[0038] Sodium fluoride and magnesium fluoride are mixed as molten salt, sodium fluoride accounts for 60% of the total mass of the molten salt, and magnesium fluoride accounts for 40% of the total mass of the molten salt.
[0039] Add magnesium oxide accounting for 3% of the total mass of sodium fluoride and magnesium fluoride into the molten salt and mix evenly.
[0040] Put the molten salt containing magnesium oxide in the electrolytic cell, heat the molten salt in the electrolytic cell to 900°C, the two poles start to be energized, and the anode current density during electrolysis is 0.5A / cm 2 , the electrolysis time is 0.4h; then the corundum crucible and cathode filled with magnesium-nickel alloy at the bottom of the electrolytic cell are taken out, put into a new cathode and corundum crucible, and through chemical analysis, a magnesium-nickel alloy with a metal magnesium mass content...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com