Titanium catalyst and preparation method and application thereof
A titanium catalyst and catalyst technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problems of unsuitable polymerization reaction catalysts, difficulty in obtaining high viscosity and low activity and other problems, to achieve the effect of improving catalytic activity, short esterification reaction and polymerization reaction time, and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
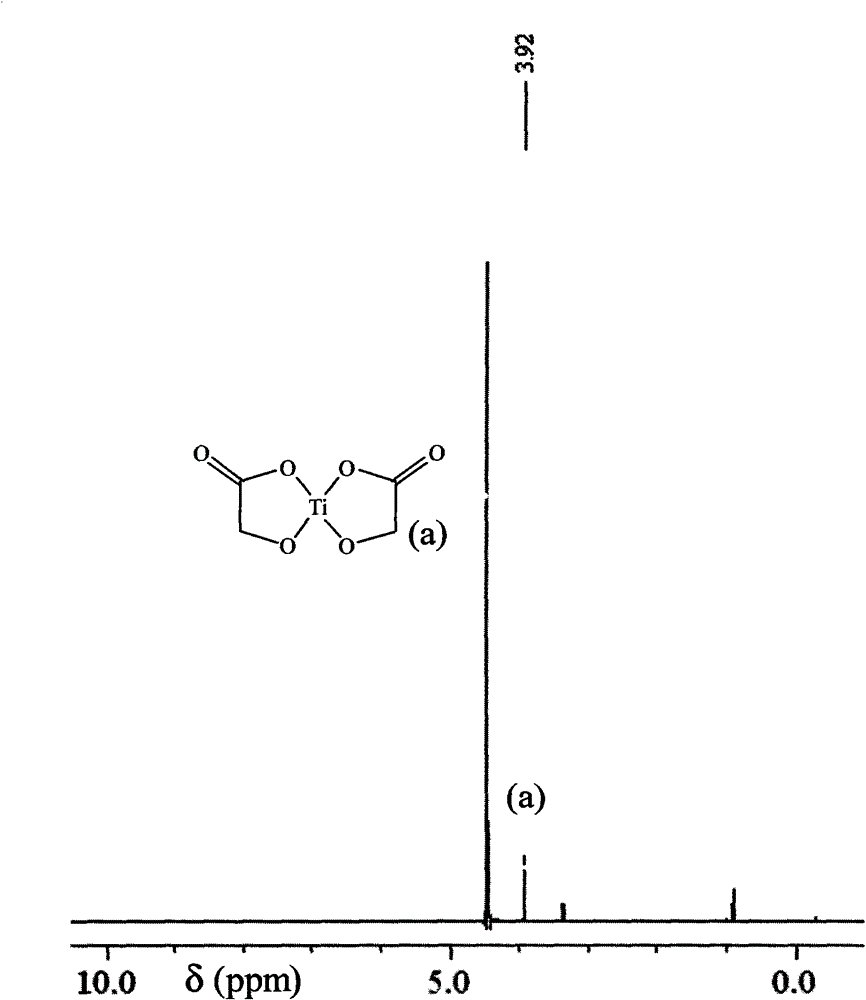
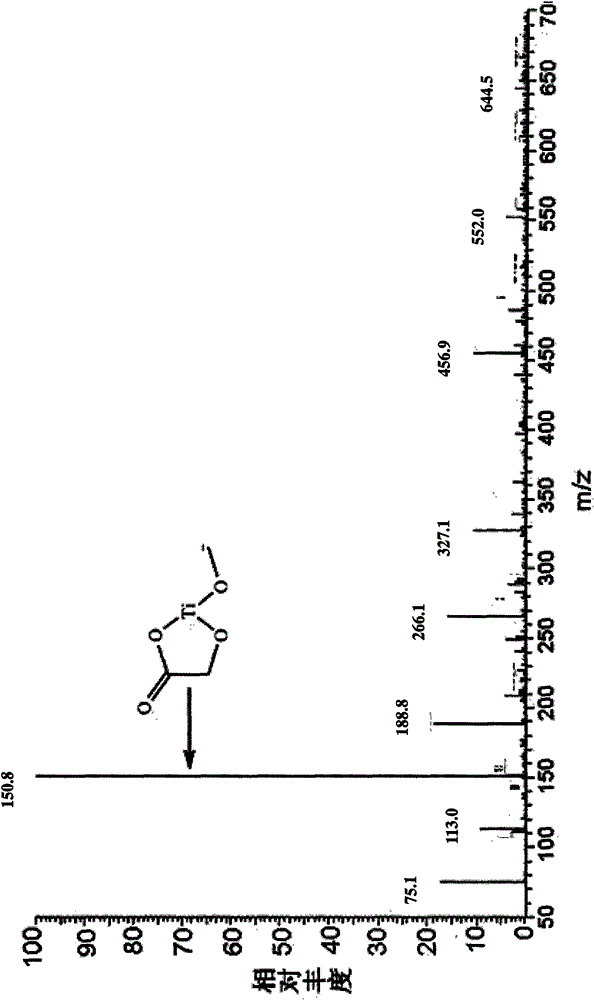
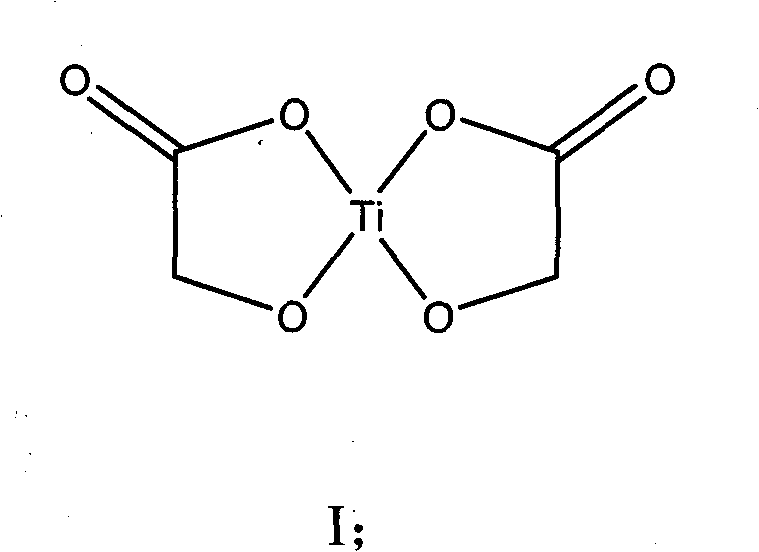
[0023] Weigh 18.38 grams of α-glycolic acid (Sinopharm Chemical Reagent Co., Ltd., chemically pure), put it into a 250-ml round-bottomed single-necked flask, measure 60 mL of tetrahydrofuran and pour it into the flask, magnetically stir to dissolve α-glycolic acid, and heat up to At 60°C (oil bath temperature, the same below), add 40.84g of butyl titanate (Tianjin Fuchen Chemical Reagent Factory, chemically pure) dropwise with a constant pressure dropping funnel, a large amount of white precipitates are produced, and the dropwise addition is completed in 1 hour. Heat up to tetrahydrofuran reflux, continue to stir and react for 2.5 hours, cool to room temperature, carefully filter with suction, wash with a small amount of absolute ethanol several times, drain, dry in a blast oven at 70°C for several hours, and weigh 22.57g , the yield was 96%.
Embodiment 2
[0025] Put 91.46 grams of α-glycolic acid (Shanghai Aladdin Reagent Factory, purity 99.5%) into a 1000mL three-necked flask, then pour 150mL of absolute ethanol (analytical grade), stir magnetically, heat up to 65°C, and wait for α-glycolic acid After it was completely dissolved, 204.17 g of butyl titanate (Tianjin Fuchen Chemical Reagent Factory, chemically pure) was added dropwise through a constant-pressure dropping funnel. A large amount of white precipitates were produced, and the addition was completed in about 1 hour. Continue to stir and react at this temperature for 2.5 hours, cool to room temperature, carefully suction filter, wash 3 times with a small amount of absolute ethanol, drain, dry in a blast oven at 70°C for several hours, and weigh 106.26 grams. The yield is 90%.
Embodiment 3
[0027] Weigh 91.30 grams of α-glycolic acid (Shanghai Aladdin Reagent Factory, purity 99.5%) in a 1000mL three-necked flask, then pour 150mL of absolute ethanol (analytical grade), and stir magnetically. After the α-glycolic acid is completely dissolved, Add 204.04 g of butyl titanate (Tianjin Fuchen Chemical Reagent Factory, chemically pure) dropwise at room temperature, and finish the dropwise addition in 3 hours. Only when more butyl titanate is added dropwise will white precipitate occur. Continue to stir and react for 2 hours at room temperature , carefully suction filtered, washed 3 times with a small amount of absolute ethanol, drained, dried in a blast oven at 90°C for 5 hours, and weighed to obtain 104.48 grams of titanium α-hydroxyacetate, with a yield of 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com