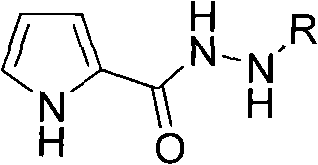
N-arylating method using pyrrole-2-hydrazide compound as ligand in aqueous phase system
A technology of hydrazides and compounds, applied in the field of chemistry, can solve problems such as long reaction time and narrow substrate adaptability, and achieve the effects of simple operation, high yield and wide application range of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the synthesis of N-p-methoxyphenylaniline
[0027]
[0028] 9.5mg (0.05mmol) CuI, 50mg (0.25mmol) ligand, 187mg (1.0mmol) p-methoxybromobenzene, 372mg (4.0mmol) aniline, 112mg (2.0mmol) KOH, 161mg (0.5mmol) TBAB, 2.0mlH 2 O was added into a 10ml microwave reaction tube and reacted at 130°C (100W) for 5 minutes. After the reaction stopped, add 10ml of water, extract with ethyl acetate (3×20ml), combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, filter, distill off the solvent under reduced pressure, and pass the obtained reaction mixture through a silica gel column Separation and purification by column chromatography [eluent: petroleum ether / ethyl acetate (20:1)] gave 156 mg of N-p-methoxyphenylaniline with a yield of 78%.
[0029] N-p-methoxyphenylaniline: 1 H NMR (300MHz, CDCl 3 ): δ7.20-7.25(m, 2H), 7.08(d, J=7.8Hz, 2H), 6.82-6.93(m, 5H), 5.49(br s, 1H), 3.82(s, 3H). 13 C NMR (75MHz, CDCl 3 ): δ155.0, 144.9...
Embodiment 2
[0031] Embodiment 2: the synthesis of 4-methoxy-N-p-methylaniline
[0032]
[0033] 4.0mg (0.05mmol) CuO, 31mg (0.25mmol) ligand, 187mg (1.0mmol) p-methoxybromobenzene, 428mg (4.0mmol) p-methylaniline, 112mg (2.0mmol) KOH, 161mg (0.5mmol) )TBAB, 2.0ml H 2 O was added into a 10ml microwave reaction tube and reacted at 130°C (100W) for 5 minutes. After the reaction stopped, add 10ml of water, extract with ethyl acetate (3×20ml), combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, filter, distill off the solvent under reduced pressure, and pass the obtained reaction mixture through a silica gel column Separation and purification by column chromatography [eluent: petroleum ether / ethyl acetate (20:1)] gave 119 mg of 4-methoxy-N-p-methylaniline with a yield of 56%.
[0034] 4-Methoxy-N-p-methylaniline: 1 H NMR (300MHz, CDCl 3 ): δ7.05(m, 4H), 6.86(m, 4H), 5.41(brs, 1H), 3.81(s, 3H), 2.31(s, 3H). 13 C NMR (75MHz, CDCl 3 ): δ154.5, 142.2,...
Embodiment 3
[0036] Embodiment 3: the synthesis of N-benzylaniline
[0037]
[0038] 95mg (0.5mmol) CuI, 35mg (0.25mmol) ligand, 157mg (1.0mmol) bromobenzene, 214mg (2.0mmol) benzylamine, 80mg (2.0mmol) NaOH, 161mg (0.5mmol) TBAB, 2.0ml H 2 O was added into a 10ml microwave reaction tube and reacted at 130°C (100W) for 5 minutes. After the reaction stopped, add 10ml of water, extract with ethyl acetate (3×20ml), combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, filter, distill off the solvent under reduced pressure, and pass the obtained reaction mixture through a silica gel column Separation and purification by column chromatography [eluent: petroleum ether / ethyl acetate (20:1)] gave 119 mg of N-benzylaniline with a yield of 65%.
[0039] N-Benzylaniline: 1 H NMR (300MHz, CDCl 3 ): δ7.17-7.41(m, 7H), 6.65-6.77(m, 3H), 4.36(s, 2H), 4.06(br s, 1H). 13 C NMR (75MHz, CDCl 3 ): δ147.9, 139.3, 129.1, 128.4, 127.3, 127.0, 117.4, 112.7, 48.2. ESI-MS...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com