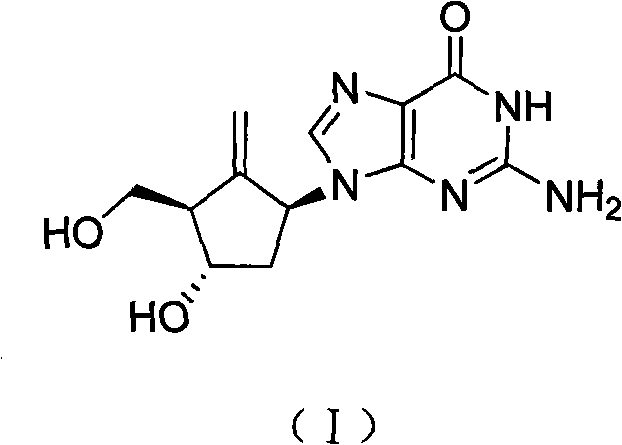
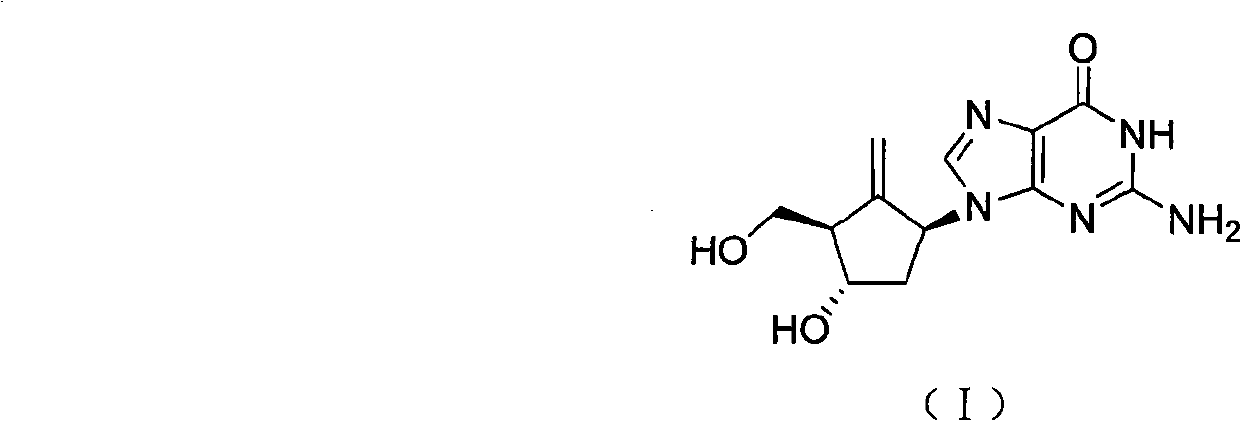
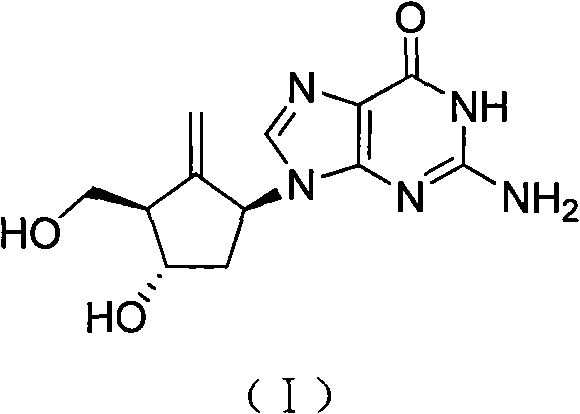
Method for preparing entecavir
A technology of entecavir and compounds, applied in the field of chemical synthesis, can solve the problems of large pollution and low yield, and achieve the effect of simple purification, high yield and low environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: preparation compound 1
[0050] Under anhydrous and oxygen-free conditions, NaH (4.023g, 60%, 100mmol) was mixed with anhydrous THF (50ml), cooled to below -10°C by ice-salt bath, and fresh cyclopentadiene (10.02ml , 100mmol), attention should be paid to degassing during the dropwise addition. After the dropwise addition, slowly rise to room temperature, let stand for more than 1h, and take the supernatant for later use.
[0051] Under anhydrous and oxygen-free conditions, the IPC 2 BH [Refer to J.Org.Chem.1982, 47, 5065-5069 for the preparation method] (100mmol) was mixed with anhydrous THF (40ml), cooled to -78°C, and set aside.
[0052] Under anhydrous and oxygen-free conditions, benzyl chloromethyl ether (20.8ml, 150mmol) was mixed with anhydrous THF (80ml), cooled to -78°C through a dry ice acetone bath, and then sodium cyclopentadiene in tetrahydrofuran was added dropwise Solution, keep the inner temperature between -30--40°C during the whole drop...
Embodiment 2
[0055] Embodiment 2: preparation compound 2
[0056] Under anhydrous and oxygen-free conditions, compound 1 (4.813g, 23.56mmol) was dissolved in anhydrous dichloromethane (40ml), and then mixed with vanadyl acetylacetonate (122mg, 0.46mmol) in anhydrous dichloromethane (10ml ) solution mixed. At 0°C, tert-butanol peroxide (5N in DCM, 9.4ml, 47.12mmol) was slowly added dropwise. After the addition was complete, the mixture was stirred overnight at room temperature.
[0057] The reaction mixture was cooled to 0° C., and a saturated solution of sodium sulfite (30 ml) was added dropwise. After the addition was complete, the mixture was stirred at room temperature until the starch-KI test paper did not change color. Separate the organic phase, extract the aqueous phase twice with water (20ml×2), combine the organic phases, wash with water (30ml), wash with saturated sodium chloride solution (30ml), and dry over anhydrous sodium sulfate. Filtration, concentration, and filtration t...
Embodiment 3
[0059] Embodiment 3: preparation compound 3
[0060] The crude product from the previous step (4.852 g) was dissolved in absolute ethanol (40 ml), and palladium on carbon (500 mg, 10% wt) was added, and hydrogenated at normal pressure overnight. Palladium carbon was removed by filtration, the filtrate was spin-dried, purified by column chromatography, and rinsed with ethyl acetate to obtain a colorless oily liquid (2.731 g, yield 88.5%, 2 steps).
[0061] Product ESI: 131.2 (M+1). 1 H NMR (CDCl 3 , 300MHz, rt): δ=3.93-3.87(m, 1H, H-3), 3.66-3.64(m, 1H, H-5), 3.63-3.64(m, 1H, H-1), 3.45(m , 1H, OCHH), 3.39(m, 1H, OCHH), 2.52(dd, 1H, J=6.5, 5.5Hz, H-2), 2.40-2.15(m, 2H, 20H), 2.09-2.05(m, 2H, H-4). TLC: Rf = 0.3 in ethyl acetate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com