Polymer with tumor organization pH responsiveness and micelle thereof
A tumor tissue and polymer glue technology, applied in the field of biological materials, polymer materials, and pharmaceutical preparations, can solve problems such as side effects and hinder the clinical application of drugs, and achieve good biocompatibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
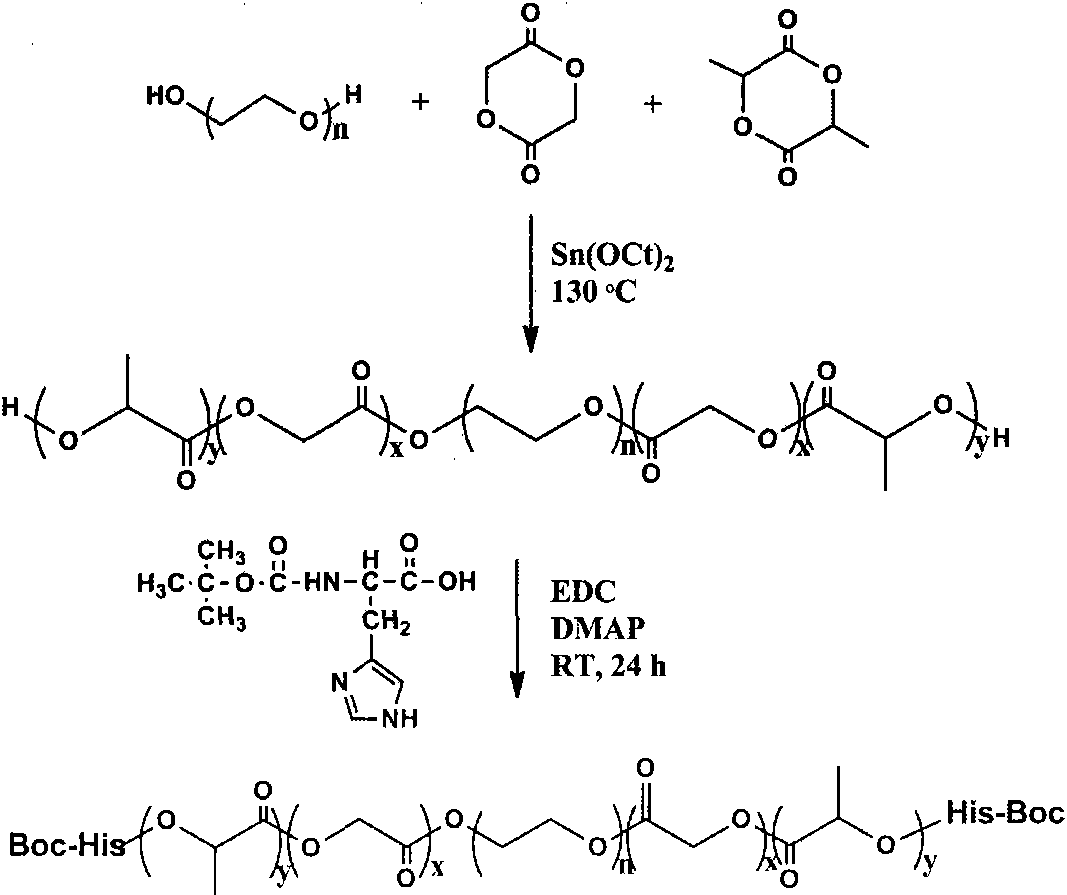
[0033] Add 30g of dihydroxypolyethylene glycol (1500) into a 500ml three-necked flask, remove water under vacuum at 130°C for 4 hours, cool to 70°C with argon, add 60g of lactide, 26mg of stannous octoate (containing a small amount of toluene), 100 ℃ for 30 minutes in vacuum, argon gas flowed, and the temperature was raised to 130 ℃ for 12 hours. After the reaction is complete, the product is poured out while it is hot, and after cooling, the product is dissolved in dichloromethane and precipitated with ether to obtain a BAB type block polymer. Take 15 g of BAB block polymer, add 3.0 g of N-Boc-histidine, 3.5 g of 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl), 4 - Dimethylaminopyrimidine (DMAP) 2.6g, dichloromethane 150ml, react at room temperature for 48 hours. Part of the solvent was removed by suspension evaporation, precipitated with ether, further washed at 80°C to remove residual unreacted substances and catalyst, and freeze-dried. Synthesis sche...
Embodiment 2
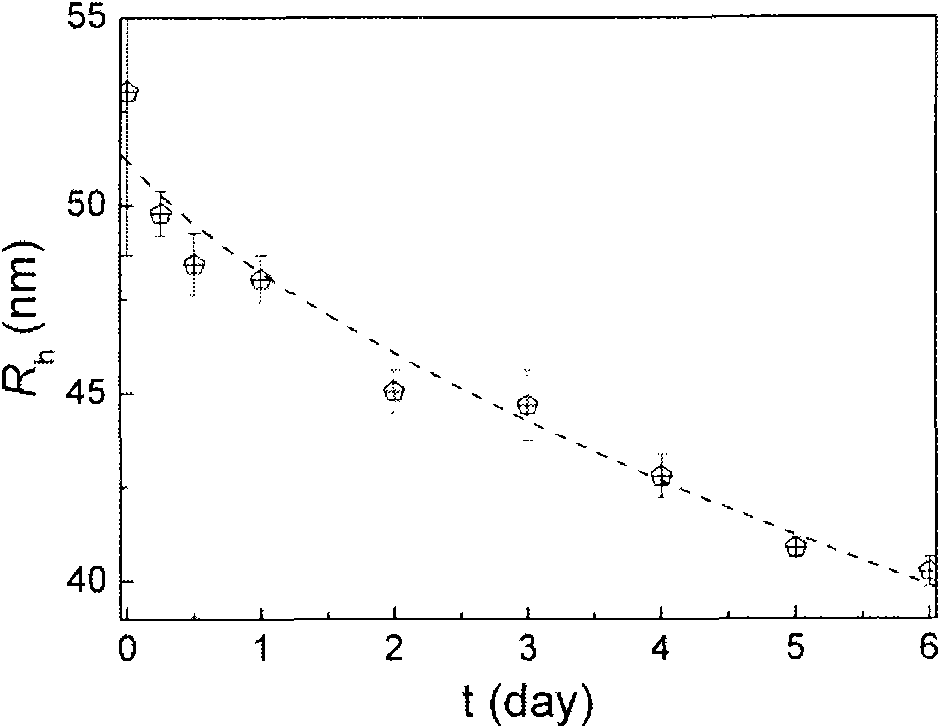
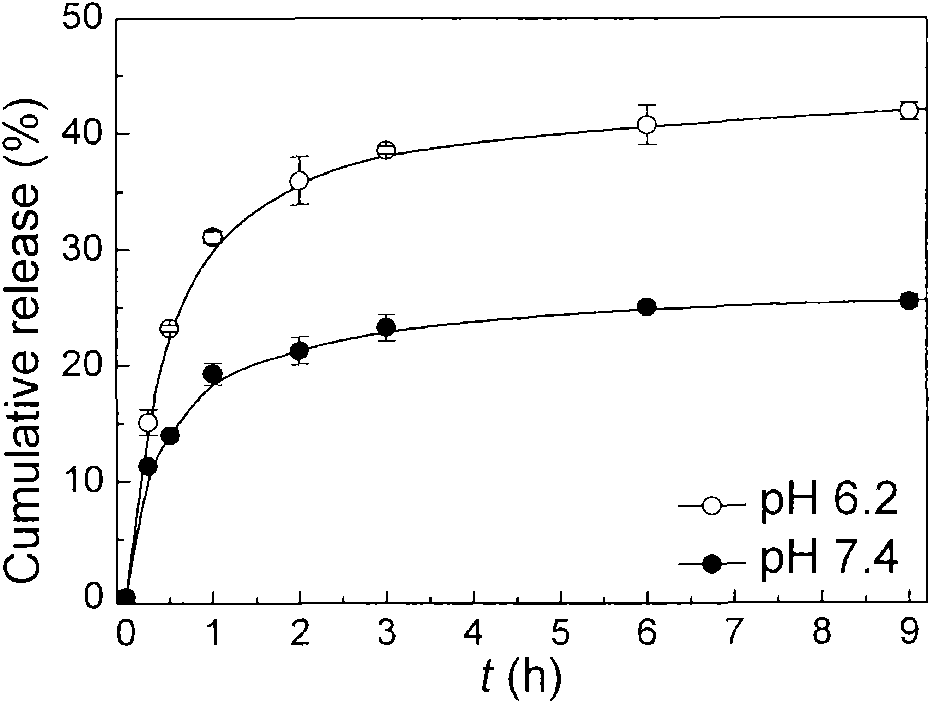
[0035] The product obtained in Example 1 was formulated into a 1 wt% solution with a phosphate buffer solution, the pH was adjusted, and filtered through a 0.8 micron filter head to obtain the required blank micellar solution. The prepared micelles can be degraded, see figure 2. 1 mg of doxorubicin hydrochloride and triethylamine were co-dissolved in methanol / chloroform (volume ratio 1:1, the same below), and the methanol / chloroform solution (2ml, 5mg / ml) containing the polymer was added dropwise, and spin Dry to form a film, add phosphate buffer for hydration, filter with a 0.8 micron filter head, and dialyze to remove free doxorubicin to obtain micelles loaded with doxorubicin. The release rate of drug-encapsulated micelles is different between the normal pH (7.4) of the human body and the pH (6.2) of the extracellular fluid in tumor tissue, see image 3 . The human breast cancer cell line MDA-MB-435 and cell culture medium were cultured in a six-well cell culture plate ...
Embodiment 3
[0037] Add 30g of dihydroxypolyethylene glycol (1500) into a 500ml three-necked flask, remove water under vacuum at 130°C for 4 hours, cool to 70°C with argon, add 50g of lactide, 10g of glycolide, 26mg of stannous octoate (solution in toluene), evacuate at 100°C for 30 minutes, pass argon, and raise the temperature to 130°C for 12 hours. After the reaction is complete, the product is poured out while it is hot, and after cooling, the product is dissolved in dichloromethane and precipitated with ether to obtain a BAB type block polymer. Take 10 g of BAB block polymer, add 1.8 g of N-Boc-histidine, 1.7 g of 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl), 4 - Dimethylaminopyrimidine (DMAP) 1.3g, dichloromethane 150ml, react at room temperature for 48 hours. Part of the solvent was removed by suspension evaporation, precipitated with ether, further washed at 80°C to remove residual unreacted substances and catalyst, and freeze-dried. The obtained product w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com