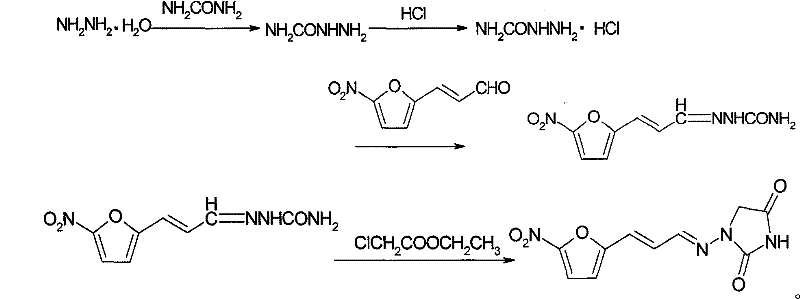
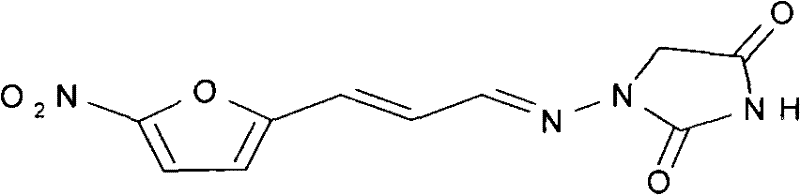
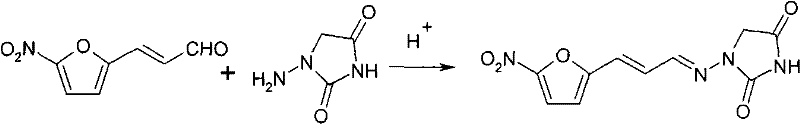
A kind of synthetic method of 1-((3-(5-nitro-2-furyl) allyl) amino)hydantoin
A technology for nitrofuran acrolein and a synthesis method, which is applied in the direction of organic chemistry and the like, can solve the problems of pressure, poor atom economy, low yield, etc. The effect of improving the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] 1. Put 100kg of 50% hydrazine hydrate solution and 90kg of urea into a 500L reactor, dissolve, stir, and heat to 100°C for reflux reaction for 6h. After the reflux reaction is completed, adjust the pH value to between 3-4 with concentrated hydrochloric acid, and then add 133 kg of 5-nitrofuran acrolein (50%, w / w) dropwise in ethanol solution. The reaction was vigorously stirred for 3 hours, heated to 80° C. for reflux reaction for 3 hours, cooled, filtered and dried to obtain 162 kg of solid product 5-nitrofuran acrolein semicarbazone. Based on 5-nitrofuran acrolein, the yield is 90%.
[0049] 2. In a 2000L reaction kettle, add 224 kg of the product 5-nitrofuran acrolein semicarbazone obtained in the previous step and 448 kg of ethanol, and stir. At the same time, 122.5 kg of ethyl chloroacetate and 1000 L of sodium ethoxide ethanol solution (2.0 mol / L) were slowly added dropwise, and the rate of addition was controlled to keep the material refluxed at a reflux tempera...
Embodiment 2
[0051] 1. Put 100kg of 50% hydrazine hydrate solution and 180kg of urea into a 500L reactor, dissolve, stir, and heat to 100°C for reflux reaction for 2 hours. After the reflux reaction is completed, adjust the pH value to between 4-5 with concentrated hydrochloric acid, and then add 167 kg of 5-nitrofuran acrolein (30%, w / w) dropwise in ethanol solution, and the dropwise addition is completed within 3 hours. The reaction was vigorously stirred for 1 hour, heated to 80° C. for reflux reaction for 0.5 hours, cooled, filtered and dried to obtain 190 kg of solid product 5-nitrofuran acrolein semicarbazone. Based on 5-nitrofuran acrolein, the yield is 85%.
[0052] 2. In a 2000L reaction kettle, add 224 kg of the product 5-nitrofuran acrolein semicarbazone obtained in the previous step and 448 kg of ethanol, and stir. At the same time, 135 kg of ethyl chloroacetate and 1000 L of ethanol solution of sodium ethoxide (3.0 mol / L) were slowly added dropwise, and the rate of addition w...
Embodiment 3
[0054] 1. Put 100kg of 50% hydrazine hydrate solution and 120kg of urea into a 500L reactor, dissolve, stir, and heat to 100°C for reflux for 4 hours. After the reflux reaction is completed, adjust the pH value to between 3-4 with concentrated hydrochloric acid, then add 133 kg of 5-nitrofuran acrolein in ethanol solution (40%, w / w) dropwise, and the dropwise addition is completed within 1.5 h. The reaction was vigorously stirred at room temperature for 2 hours, heated to 80° C. for reflux reaction for 2 hours, cooled, filtered and dried to obtain 170 kg of solid product 5-nitrofuran acrolein semicarbazone. Based on 5-nitrofuran acrolein, the yield is 95%.
[0055] 2. In a 2000L reaction kettle, add 224 kg of the product 5-nitrofuran acrolein semicarbazone obtained in the previous step and 448 kg of ethanol, and stir. At the same time, 125 kg of ethyl chloroacetate and 1000 L of ethanol solution of sodium ethoxide (2.4 mol / L) were slowly added dropwise, and the rate of additi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com