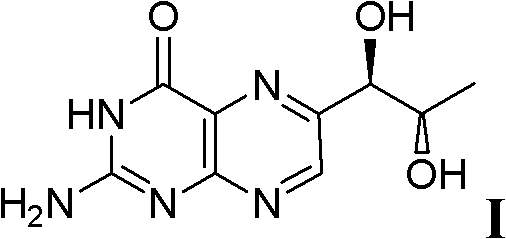
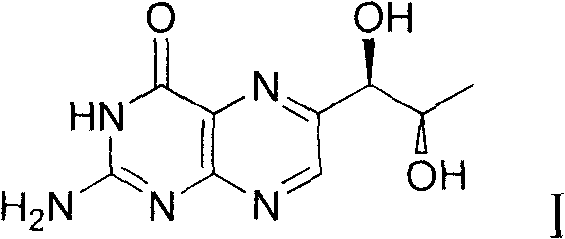
Preparation method of L-erythro biopterin
A technology of biopterin and erythrotype, which is applied in the field of preparation of L-erythrotype biopterin, can solve the problems of strong odor and long reaction cycle, and achieve the effect of short cycle, easy operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
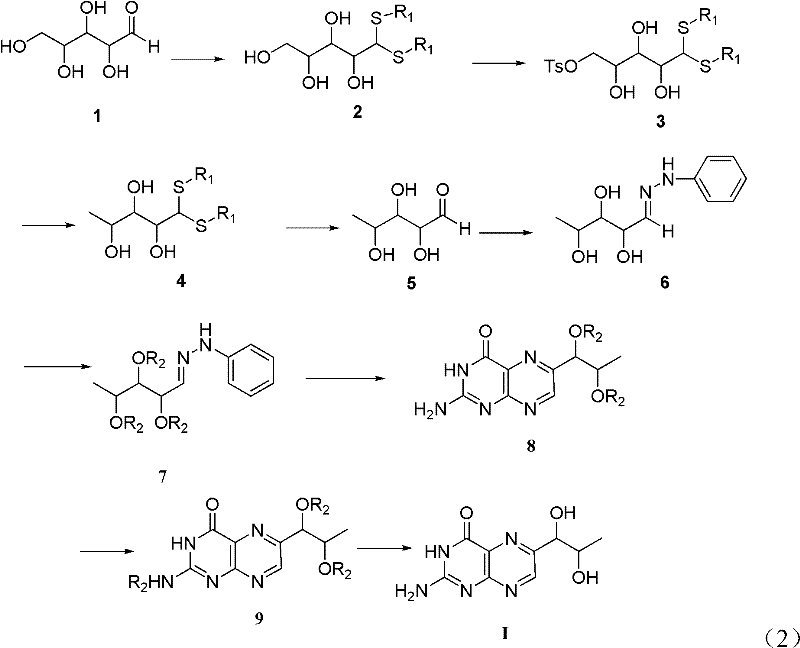
[0039] The preparation method of L-erythrotype biopterin, its specific preparation steps are as follows:
[0040] 1) Dissolve 10g of L-(+)-arabinose 1 in 20mL of concentrated hydrochloric acid, stir, then add 18mL of n-dodecyl mercaptan, stir, acetal reaction occurs, and the reaction time is 18 hours; extracting, drying the extract with magnesium sulfate, and filtering to remove the magnesium sulfate after drying to obtain a filtrate;
[0041] 2) Cool the filtrate obtained in step 1) to 0°C, then add 10mL triethylamine, then add 12.6g p-toluenesulfonyl chloride, stir, and the reaction time is 24 hours; after the reaction is completed, use saturated aqueous sodium bicarbonate Washing three times, separating the layers, drying the organic phase with magnesium sulfate, filtering, and then distilling off the solvent under reduced pressure to obtain the product 3';
[0042] 3) Dissolve the product 3' obtained in step 2) in 200 mL of tetrahydrofuran, cool to 0° C., then add 2.2 g o...
Embodiment 2
[0055] The preparation method of L-erythrotype biopterin, its specific preparation steps are as follows:
[0056] 1) Dissolve 10g of L-(+)-arabinose 1 in 20mL of concentrated hydrochloric acid, stir, then add 18mL of n-dodecyl mercaptan, stir, acetal reaction occurs, and the reaction time is 18 hours; extracting, drying the extract with magnesium sulfate, and filtering to remove the magnesium sulfate after drying to obtain a filtrate;
[0057] 2) Cool the filtrate obtained in step 1) to 0°C, then add 10mL triethylamine, then add 12.6g p-toluenesulfonyl chloride, stir, and the reaction time is 24 hours; after the reaction is completed, use saturated aqueous sodium bicarbonate Washing three times, separating the layers, drying the organic phase with magnesium sulfate, filtering, and then distilling off the solvent under reduced pressure to obtain the product 3';
[0058] 3) Dissolve the product 3' obtained in step 2) in 200mL DMSO, cool to 0°C, then add 2.2g sodium borohydride,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com