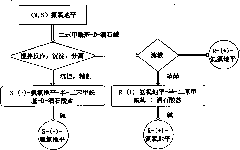
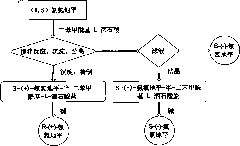
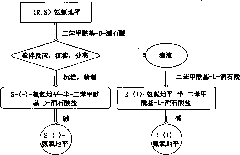
Method for preparing S-(-)-amlodipine and R-(+)-amlodipine by chirally resolving racemic amlodipine
A technology for amlodipine and chiral separation, applied in chemical instruments and methods, organic racemization, organic chemical methods, etc., can solve the problems of large amount of separation solvent, cumbersome operation, solvent residue, etc., and achieve product yield High, good optical purity, the effect of a small amount of solvent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Preparation of S-(-)-amlodipine-semi-dibenzoyl-D-tartrate
[0035] Dissolve 5g (0.0122mol) (R, S) amlodipine in 20mL of absolute ethanol, heat to dissolve, add 1.1g (0.0031mol) of dibenzoyl-D-tartaric acid dissolved in 10mL of absolute ethanol, stir React until there is a large amount of precipitation, and then continue the crystallization reaction at room temperature for 4 hours, put it in the refrigerator overnight, and filter to obtain S-(-)-amlodipine-semi-dibenzoyl-D-tartrate. Refined with ethanol and vacuumed at 50°C for 2 hours to obtain 2.89 g of S-(-)-amlodipine-semi-dibenzoyl-D-tartrate, with a yield of 80.4%. The diastereomeric excess (d.e) was determined to be 99.08% by chiral HPLC. M.p. is 117~118℃, measured value of elemental analysis: C59.16%, H5.53%, N4.67%; calculated value: C 20 h 25 N 20 o 5 Cl 0.5(C 18 h 14 o 8 ): C59.23%, H5.49%, N4.76%. 1 HNMR agrees with the molecular structure, see attached Figure 5 .
Embodiment 2
[0036] Example 2 Preparation of S-(-)-amlodipine-semi-dibenzoyl-D-tartrate
[0037] Dissolve 5g (0.0122mol) (R, S) amlodipine in 20mL of absolute ethanol, heat to dissolve, add 10mL of absolute ethanol dissolved with 2.2g (0.0061mol) of dibenzoyl-D-tartaric acid, stir React until there is a large amount of precipitation, then continue the crystallization reaction at room temperature for 4h, and then crystallize at -10°C for 4h, filter to obtain S-(-)-amlodipine-semi-dibenzoyl-D-tartrate, the crude product is used Refined with absolute ethanol and vacuumed at 50°C for 2 hours to obtain 3.11 g of S-(-)-amlodipine-hemi-dibenzoyl-D-tartrate, with a yield of 86.52%. The diastereomeric excess (d.e) was determined to be 98.54% by chiral HPLC.
Embodiment 3
[0038] Example 3 Preparation of S-(-)-amlodipine-semi-dibenzoyl-D-tartrate
[0039] Dissolve 5 grams (0.0122mol) of (R, S) amlodipine in 40ml of dehydrated ethanol, heat to dissolve, add 10ml of dehydrated ethanol dissolved with 2.2 grams (0.0061mol) of dibenzoyl-D-tartaric acid, Following the operation according to Example 2, 2.45 grams of S-(-)-amlodipine-semi-dibenzoyl-D-tartrate was obtained, with a yield of 68.2%. The diastereomeric excess (d.e) determined by chiral HPLC was 98.36%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com