Preparation method of anti-tumour medicine intermediate 10-deacetylbacctin III
A technology for antitumor drugs and intermediates, applied in the field of medicine, can solve the problems of harsh reaction conditions, low product yield and purity, unsuitable for industrial production, etc., and achieve the effects of high product purity and yield and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The cephalomannine used in the present invention can be isolated from Taxus plants, or is a by-product produced during the paclitaxel extraction process.
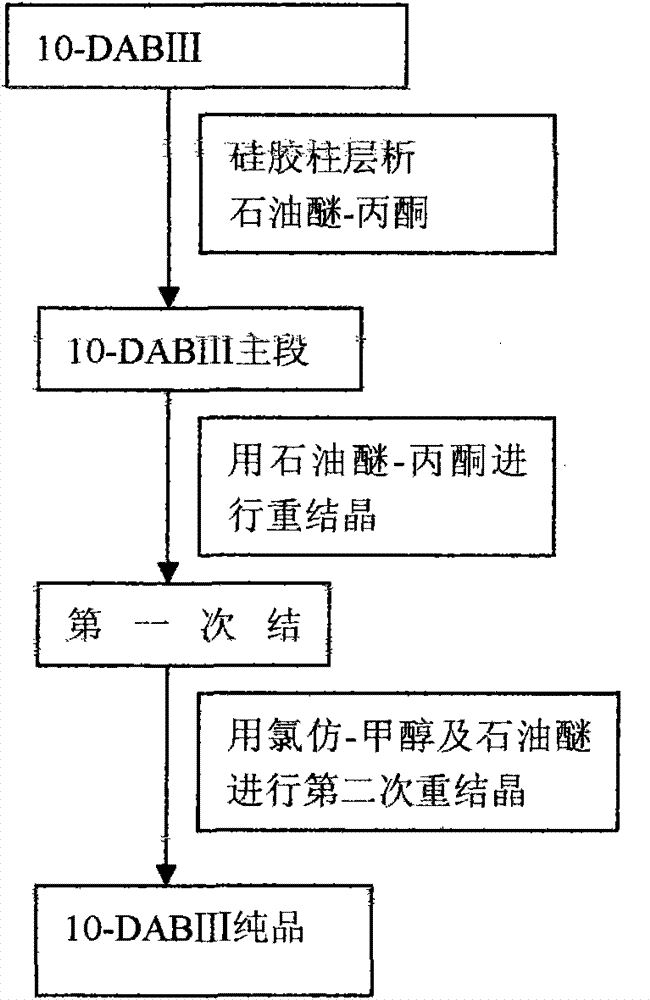
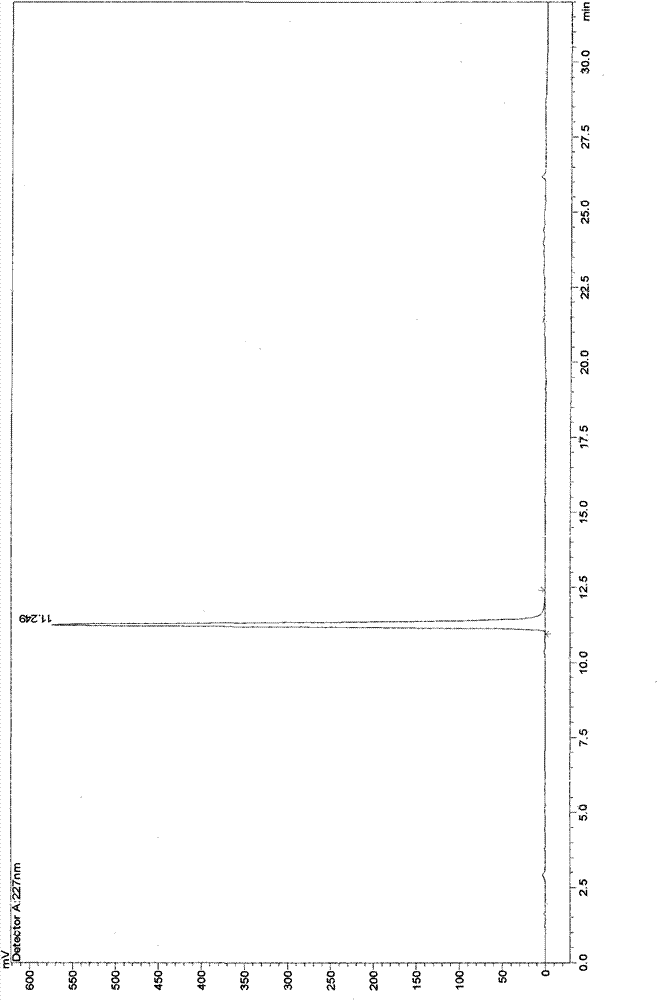
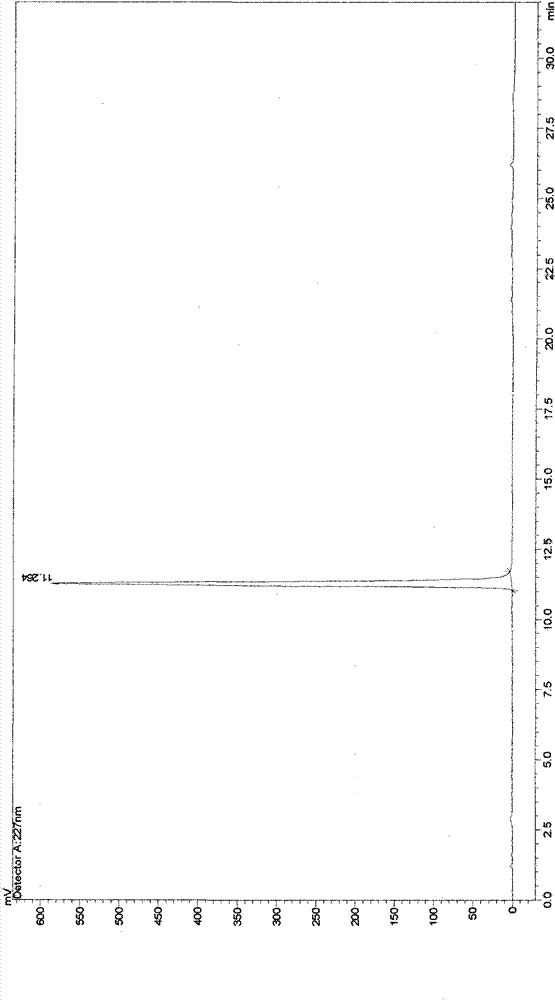
[0043] Take 10g of cephalomannine and dissolve it in 100ml of methanol, add 132ml of 80% hydrazine hydrate and stir at 30°C for 1.5 hours, add 150ml of saturated aqueous ammonium chloride solution, stir at 30°C for 3 hours, add 382ml of ethyl acetate, stir, and let stand 2 hours, separate layers, take the organic layer, and extract the aqueous layer twice with 382ml ethyl acetate respectively, combine the ethyl acetate layers, and extract once with 382ml saturated brine to obtain the ethyl acetate extract, and the ethyl acetate extract is reduced Concentrate under reduced pressure, purify the concentrate through a 200-300 mesh silica gel column, and elute with petroleum ether-acetone (v:v=3:1) to obtain the main part of 10-DABIII, which is heated and dissolved with 120ml of acetone at 60°C , add sherwood oil afterwar...
Embodiment 2
[0045] Take 10g of cephalomannine and dissolve it in 150ml of ethanol, add 130ml of 80% hydrazine hydrate, stir at 30°C for 1.5 hours, add 150ml of saturated aqueous ammonium chloride solution, stir at 30°C for 3 hours, add an equal volume of ethyl acetate, stir, and statically Stand for 2 hours, separate layers, take the organic layer, extract the aqueous layer twice with an equal volume of ethyl acetate, combine the ethyl acetate layers, and extract once with 432ml of saturated brine to obtain an ethyl acetate extract, which is extracted with ethyl acetate The liquid was concentrated under reduced pressure, and the concentrate was purified by a 200-300 mesh silica gel chromatography column, and eluted with petroleum ether-acetone (v:v=3:1) to obtain the main part of 10-DABIH, which was washed with 120ml of acetone at 60°C. Heat to dissolve, then add n-hexane until crystals precipitate, let stand for 24 hours, filter, heat and dissolve the filter cake with 95ml chloroform meth...
Embodiment 3
[0047] Take 10g of cephalomannine and dissolve it in 120ml of ethanol, add 135ml of 80% hydrazine hydrate, stir at 30°C for 1.5 hours, add 150ml of saturated aqueous ammonium chloride solution, stir at 30°C for 3 hours, add an equal volume of ethyl acetate, stir, and statically Stand for 2 hours, separate layers, take the organic layer, and extract the aqueous layer twice with equal volume of ethyl acetate, combine the ethyl acetate layers, and extract once with 402ml saturated brine to obtain ethyl acetate extract, and extract with ethyl acetate The liquid was concentrated under reduced pressure, and the concentrate was purified by 200-300 mesh silica gel chromatography, and eluted with petroleum ether-acetone (v:v=3:1) to obtain the main part of I0-DABIII, which was washed with 120ml of acetone at 60°C Heat to dissolve, then add cyclohexane until crystals precipitate, let stand for 24 hours, filter, heat and dissolve the filter cake with 82ml chloroform methanol (1:1) at 60°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com