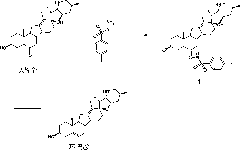
Preparation method of cyclopamine
A technology for cyclopamine and the next step, which is applied in the field of preparation of cyclopamine using filimonium as a raw material, can solve the problems of non-stereoselectivity, complex synthesis process, and yield less than 1%, etc. Achieve the effect of simple operation, simple purification and cheap reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
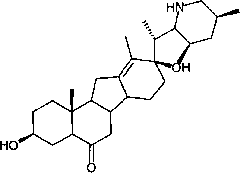
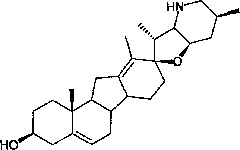
[0038] Dissolve 500 mg of fritillary octane (obtained by the method in Example 3) and 315 mg of p-toluenesulfonyl hydrazide in 50 ml of methanol, add 0.1 ml of concentrated hydrochloric acid, stir at room temperature for 1 h, add 0.1 ml of concentrated hydrochloric acid, continue stirring for 2 h, and monitor by TLC. The response is complete. Concentrate under reduced pressure at 20-30°C to dryness, add 30ml of water, extract 50ml*3 with ethyl acetate, and concentrate under reduced pressure at 20-30°C to dryness to obtain crude white solid intermediate 1 tosylhydrazone. The crude intermediate 1 was dissolved in 70ml of toluene, 700mg of lithium hydride was added, heated and stirred at reflux (120°C) for 5 hours, and the reaction was complete as monitored by TLC. Cool to 20-30°C, filter and wash with ethyl acetate (30ml). The filtrate was concentrated to dryness under reduced pressure at 20-30°C to obtain the crude cyclopamine product, which was dissolved in 20ml of boiling ac...
Embodiment 2
[0042] Dissolve 100 mg of fritillary octane (obtained by the method in Example 3) and 63 mg of p-toluenesulfonyl hydrazide in 15 ml of methanol, add 70 mg of p-toluenesulfonic acid, stir at 40° C. for 5 h, and monitor the completion of the reaction by TLC. Concentrate under reduced pressure at 20-30°C to dryness to obtain crude intermediate 1 tosylhydrazone as a white solid. The crude product 1 was dissolved in 20ml of toluene, 220mg of sodium hydride was added, heated and stirred at reflux (120°C) for 5 hours, and the reaction was complete as monitored by TLC. Cool to 20-30°C, filter and wash with ethyl acetate (10ml). The filtrate was concentrated under reduced pressure at 20-30°C to obtain the crude cyclopamine product, which was dissolved in 5ml of boiling acetone, and 6ml of petroleum ether was slowly added until turbidity appeared, then left to cool to room temperature (15°C) , a large amount of white solid was separated out, and 84 mg of white solid cyclopamine was obt...
Embodiment 3
[0046] The Chinese herbal medicine fritillary fritillaria was crushed through a 80-100 mesh sieve, weighed 20kg, extracted 3 times (100Lx3) with 300L of 95% industrial ethanol at 20-30°C, combined the extracts, concentrated under reduced pressure with a vacuum pump, and the temperature of the water bath was 40°C, after removing the solvent, 1 kg of ethanol extract was obtained (extract density M:M=1:20). The ethanol extract was extracted 3 times with chloroform (10Lx3), the combined extracts were vacuum filtered, and concentrated under reduced pressure with a vacuum pump (water bath temperature was 30°C) to obtain 513g of chloroform extract (extract density M:M=1:40). The chloroform extract is separated with a normal phase silica gel column (300-400 mesh, Qingdao Marine) mobile phase as petroleum ether-acetone (2:1-1:1) (0.5% triethylamine) and 100ml of acetone at 20-25°C Recrystallization obtained fritillaria 1.5g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com